Case Studies: 2020 Vol: 26 Issue: 3
Generic Drug Shortages: A Case for Continuous Manufacturing
Stephanie Miller, Quinnipiac University
Christopher Hodgdon, Quinnipiac University
Angela Mattie, Quinnipiac University
Abstract
The primary subject matter of this case is continuous manufacturing and how it relates to the production of generic drugs. Students in accounting, management, and supply chain programs will benefit from the case. The case is appropriate for senior-level undergraduate courses and for first-year graduate students. This case can be taught within three hours of classroom time. Students will need two to three hours of outside preparation, depending upon their familiarity with supply chain issues and batch and continuous manufacturing.
Keywords
Generic Drugs, Continuous Manufacturing, Supply Chain, Batch Manufacturing
Introduction
Prescription drug expenditures have risen steadily over the past three decades in the United States, and high brand-name drug prices have often captured headlines. However, the media and academic literature alike have given far less attention to another facet of the drug pricing problem - extremely low prices for generic drugs (Henderson & Hooper, 2019). Over the past decade, as brand-name drug prices have soared, generic drug prices have steadily decreased relative to the consumer price index (Peter G. Peterson Foundation, 2018). Manufacturers have little incentive to produce generic drugs at such low profit margins (Koons, 2018). With few manufacturers producing a given drug, there is a significant risk of supply shortages, and the supply chain is vulnerable to external events. The FDA cites lack of incentive to produce less profitable drugs and supply chain issues as two key causes of drug shortages (FDA, 2020).
The recent COVID-19 pandemic demonstrates this problem with alarming clarity. In addition to shortages of ventilators and personal protective equipment, the FDA has reported shortages of key antibiotics and ventilator-associated drugs (FDA, n.d.). Dr. Stephen Schondelmeyer, an expert on drug supply, notes that of 21 antibiotics critical for treating COVID-19 patients, 18 are generic drugs with more than 80 percent of their supply chains based in China, India, or Italy, where COVID-19 created significant production disruptions (Sheikh, 2020). The problem of drug shortages is not new. Of 156 drugs likely to increase patient death rates if they are even briefly unavailable, 60 were in shortage even before the pandemic (Ross, 2020).
Generic drugs make up 90% of all prescriptions in the United States (National Conference of State Legislators, 2019). Thus, problems arising from the pricing and supply of generic drugs have the potential to affect millions of patients. Through continuous manufacturing, these drugs can be produced more profitably (Executive Office of the President of the United States, 2016), and production can be modernized to respond to existing and emerging pathogens (Witten, 2018).
Dealing with the Issue: The Case of Jazzy Pharmaceuticals1
Every Monday morning Jazzy Pharmaceuticals executives begin with a weekly senior leadership meeting where they discuss operational issues and strategy. This Monday is no different. Geoffrey Starling, VP of Process Science & Advanced Analytics, is preparing for the weekly meeting when some recent media and field reports catch his eye. Premier, an antiviral medication used to treat HIV, is important to society, simple to make, and very stable (Bell, 2018). It makes sense to explore an alternative for manufacturing Premier to protect against shortages, since patients depend on this medication to maintain health. Mr. Starling, however, realizes this will not be an easy sell to his colleagues. Jazzy and the entire pharmaceutical industry rely on batch manufacturing to produce drugs. Mr. Starling sets out to build an argument for continuous manufacturing for the senior leadership meeting.
Batch Manufacturing
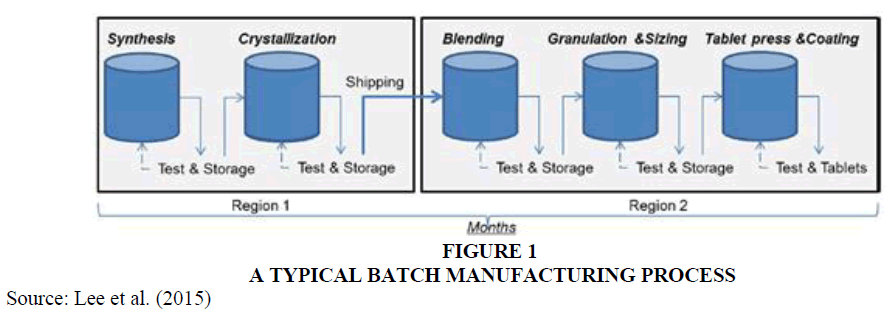
Most pharmaceuticals are manufactured using batch processing (Myerson, 2015), a multi-step process in which ingredients are processed in separate containers. At each step in the process, quality is tested before the product moves to the next step. This continuous stopping, testing, and resuming production can be time consuming and sometimes results in product wastage and unsatisfactory quality (Toro, 2019). Inefficiencies in batch processing (e.g., time, waste, contamination, recalls) cost the pharmaceutical manufacturing industry approximately $50 billion a year (General Kinematics, 2017). Inefficient production processes also leave manufacturers ill-equipped to handle demand spikes and supply disruptions, both of which arose during the COVID-19 pandemic Figure 1.
The Case for Continuous Manufacturing
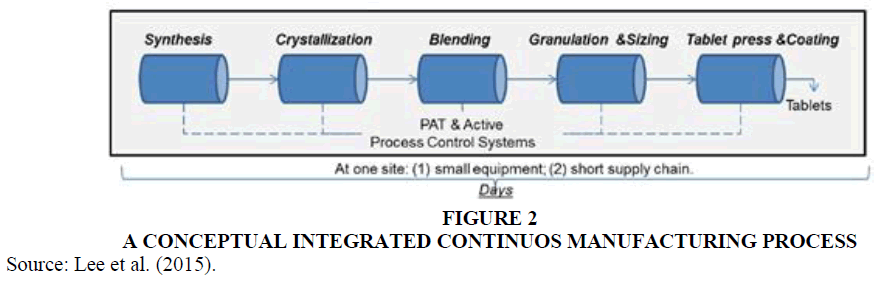
Continuous manufacturing is an alternative to batch processing in which multiple steps are integrated into a single system. Ingredients are continuously fed into the manufacturing system and transformed into output with limited shutdowns. The benefits of continuous manufacturing are numerous. Manufacturing processes are streamlined, resulting in greater manufacturing efficiency and the ability to reduce cycle times significantly (Executive Office of the President of the United States, 2016). Through advanced control systems, real-time product testing, and limited stoppages, continuous manufacturing can improve product quality and consistency over batch manufacturing (Snyder, 2016); Executive Office of the President of the United States, (2016). Continuous manufacturing generally requires a smaller manufacturing footprint, reduces need for storage and testing, and generates less product waste, all of which can make it an environmentally friendly alternative to batch processing and dramatically reduce manufacturing costs (Price, 2018). Lastly, production can be easily and quickly adjusted in response to market demands (Lee, 2019). The FDA estimates that a drug that takes up to a month to produce with batch processing could be produced in just one day with continuous manufacturing (McCarthy, 2019).
Pharmaceuticals valued at $393 million were produced by continuous manufacturing in 2018, and the market is expected to expand to $855 million by 2026 (NASDAQ, 2020). However, the pharmaceutical industry has been slow to adopt continuous manufacturing, primarily because of the high costs of converting existing batch processing systems and the fear of introducing new risks into the drug production process (Pagliarulo, 2018). In addition, the highly regulated nature of the pharmaceutical industry means that companies must obtain approval from the FDA before switching from batch processing to continuous manufacturing. Mr. Starling knows a solid business case is needed to switch to continuous manufacturing Figure 2.
The Business Case
Mr. Starling knows that Jazzy’s corporate culture is one that supports innovation. Converting to continuous manufacturing would take much time and effort and require cooperation within and outside the organization. New equipment may need to be purchased, and elements from different vendors (e.g., feeders, blenders, etc.) would have to be integrated mechanically and then electronically. Once the process is outlined, the company would need to seek approval from the FDA and train employees in the new process. Although the initial investment might be large (the estimated conversion cost is $10 million), Mr. Starling believes the subsequent cost savings would be substantial. To illustrate, he prepares a hypothetical analysis of the direct labor, direct material, and manufacturing overhead savings the company would experience from continuous manufacturing with a hypothetical annual production of 200,000 kilograms of Premier. His analysis shows the following (De Belder, 2016) Table 1.
| Table 1 Batch to Continuous Manufacturing | |||
| Costs | Batch Processing | Continuous Manufacturing | Savings |
| Direct Labor | $1,164,000 | $832,600 | $331,400 |
| Direct Material | 375,000 | 144,000 | 231,000 |
| Manufacturing Overhead | |||
| Indirect Labor | 189,700 | 23,713 | 165,987 |
| Other Overhead | 440,000 | 220,000 | 220,000 |
| Total Annual Savings | $948,387 | ||
Jazzy Moves from Batch to Continuous Manufacturing
Mr. Starling and his team successfully convinced senior management to switch to continuous manufacturing for Premier. In April 2016, in a first-of-its-kind decision, the FDA granted Jazzy Pharmaceuticals permission to begin producing the drug using continuous manufacturing (Brennan, 2016). Overall, the benefits of the change were substantial. Chemical waste was reduced by 33%, the physical footprint was reduced by over half, labor hours were reduced by 70%, and cycle time was reduced from 30 days to five days (C.M. Pharma, 2019).
The FDA has since promoted the use of continuous manufacturing for pharmaceuticals. The agency believes the small footprint of continuous manufacturing systems will allow for localized manufacturing, which may shorten the drug supply chain and eventually result in lower drug prices for consumers and fewer drug shortages (Gottlieb & Woodcock, 2019).
Recommendations for Teaching Approaches
This case can be used in courses such as managerial accounting, healthcare management, supply chain, economics, or general management. The case was designed to emphasize the following learning objectives:
1. Introduce students to continuous manufacturing as it relates to the production of generic drugs
2. Identify lesser known aspects of the issues surrounding pharmaceutical prices, namely low generic drug prices and drug shortages
3. Apply business principles and critical thinking skills to analyze a proposed solution for generic drug shortages
Depending on the students’ familiarity with generic drug costs, supply chain, and batch and continuous manufacturing, we recommend assigning two readings with the introduction of the case. We recommend the following readings:
1. Food and Drug Administration. (February 26, 2019). “FDA statement on FDA’s modern approach to advanced pharmaceutical manufacturing,” https://www.fda.gov/news-events/pressannouncements/fda-statement-fdas-modern-approach-advanced-pharmaceuticalmanufacturing
2. Myerson, A. et al. (2014) Control Systems Engineering in Continuous Pharmaceutical Manufacturing May 20–21, 2014 Continuous Manufacturing Symposium. Journal of Pharmaceutical Sciences, 104, (3), 832-839.
Students should devote three hours of preparation prior to course coverage. The following teaching approach is recommended:
1. Interactive classroom discussion (one hour): Review the issue of low-cost generic drugs and the impact of minimal profit margins on the supply of generic drugs. Review current batch manufacturing processes, then cover the example of Jazzy and its drug Premier and introduce the concept of continuous manufacturing.
2. Small group discussions (one hour): Ask students to take on the role of the head czar of a newly formed White House Pandemic Response Office. This office, among other important responsibilities, is charged with ensuring there is an adequate supply of medical equipment and pharmaceuticals in the United States supply chain. How would they solve the problem of generic drug shortages? Case questions presented with this note could be used to begin the team discussion.
3. Class debrief (one hour): Have each team report on their discussions. What are the themes and key learnings?
Discussion Questions
1. Why are low-cost generic drugs an issue? Don’t the economy and healthcare system benefit from lower-cost generic drug alternatives?
New drugs are generally protected by patents of 17 years. During this time, the company that introduces the drug often markets it at a high price, justified by the need to recoup significant research and development costs. When the patent expires, other manufacturers can begin providing a generic alternative. According to the FDA, this increased competition causes the price of the drug to decrease 20 to 70 percent on average. The resulting decreased profit provides a disincentive to manufacture generic drugs. Lack of production incentives can lead to shortages, causing patients who depend on the drug to either seek a high-cost alternative or suffer the morbidity consequences of not having needed therapeutic medication.
2. What are the benefits of continuous manufacturing over conventional batch production?
Continuous manufacturing allows manufacturers to better satisfy demand for its drugs. It shortens production time, accelerating order fulfilment and reducing production costs. Continuous manufacturing allows a company to tailor lot size to a client’s needs and thus respond to market demands in real time rather than having to anticipate demand surges. This reduces the need to keep finished goods in a warehouse, further reducing costs. Batch manufacturing requires works in process be tested before moving to the next step, thus extending production time. These hold times are eliminated with continuous manufacturing by allowing an uninterrupted production process. FDA estimates indicate some drugs using batch manufacturing can take over a month to produce. The same drug, using continuous manufacturing, can be produced in a few days. Continuous manufacturing is also potentially safer and less wasteful than batch manufacturing, since pausing and restarting the production process is associated with sub-standard batches.
3. Based on Mr. Starling’s forecasts and assuming production of 200,000 kg of Premier per year, what is the payback period for this investment in continuous manufacturing?
According to Mr. Starling’s calculations, the move to continuous manufacturing is expected to cost $10 million upfront but save $948,387 per 200,000 kg of Premier produced. If annual production remains at 200,000 kg, it will take 10.5 years (10,000,000/948,387) to recoup the initial outlay.
4. Moving from batch manufacturing to continuous manufacturing presents challenges. Identify and describe these challenges.
Regulatory Challenges. The FDA is charged with overseeing the safety, efficacy, and quality of drugs in the U.S. It has a rigorous and lengthy process to assure drugs pass safety and quality standards. Approvals for existing pharmaceuticals are tied to the production methods defined in their original submissions to the FDA. A transition from batch to continuous manufacturing requires a new submission to the FDA. No worldwide regulatory approval process for continuous manufacturing currently exists, further complicating global product launches. This lengthy and complicated regulatory approval process may push manufacturers to select a batch process for new drugs.
Workforce/Equipment/Quality Challenges. Batch processing has existed for over 50 years. A large-scale change from this conventional production method to continuous manufacturing requires new equipment, significant workforce training, and the development of novel quality and control strategies. The FDA has published guidance to address these issues, including process conditions and equipment design.
Conclusion
The first drug produced using continuous manufacturing was approved by the FDA in 2016. Since then, there has been growing attention on continuous manufacturing for drug production. However, the vast majority of drugs are still manufactured using traditional batch processing. This leaves the supply chain susceptible to disruptions that can cause shortages of critically important, lifesaving drugs. Switching to continuous manufacturing can preempt such issues. The COVID-19 pandemic made the need for innovative thinking, advance planning, and careful process design clearer than ever. Continuous manufacturing can be used to produce prescription drugs efficiently, reliably, and cost effectively. It also has applications outside pharmaceuticals and could be used to manufacture other key products. Students, as future leaders, should be educated about this emerging technology, which could allow firms and governments to better anticipate and respond to supply shocks such as those seen during the COVID-19 pandemic.
End Notes
1This case is developed for teaching purposes only to illustrate the change from batch to continuous manufacturing. It is a fictionalized account based loosely on Janssen Pharmaceutical’s successful manufacturing transition for its drug Prezista.
References
- Bell, J. (2018). “J&J still has much to explore with continuous manufacturing.” BioPharma Dive. Retrieved May 28 from https://www.biopharmadive.com/
- Brennan, Z. (2016). “FDA Allows First Switch from Batch to Continuous Manufacturing for HIV Drug.” Retrieved May 29, 2020 from raps.org
- C.M. Pharma. (2019). “Case Study: Jansen’s Prestiza Move from Batch to Continuous Manufacturing.” Retrieved May 26, 2020 from http://www.continuousmanufacturingpharma.com
- De Belder, L. (2016). “Business Case Drivers and Deployment Strategies.” Presented to ISCMP, Cambridge, MA. Retrieved May 26, 2020 from https://iscmp.mit.edu/
- Executive Office of the President of the United States (2016), Subcommittee for Advanced Manufacturing of the National Science and Technology Council. “Advanced Manufacturing: A Snapshot of Priority Technology Areas Across the Federal Government.” Retrieved May 25, 2020 from https://www.whitehouse.gov/
- Food and Drug Administration. (n.d.) “Current and Resolved Drug Shortages and Discontinuations Reported to FDA.” Retrieved May 25, 2020 from https://www.accessdata.fda.gov/
- Food and Drug Administration. (2020) “Drug Shortages: Root Causes and Potential Solutions, 2019” Retrieved May 25, 2020 from https://www.fda.gov/
- General Kinematics. (2017). “Batch Versus Continuous Pharmaceutical Manufacturing.” GK Blog. Retrieved May 26, 2020 from https://www.generalkinematics.com/
- Gottlieb, S. & J. Woodcock. (2019). “FDA statement on FDA’s modern approach to advanced pharmaceutical manufacturing.” Food and Drug Administration. Retrieved May 26, 2020 from https://www.fda.gov/
- Henderson, D.R. & C.L. Hooper, (October 31, 2019). “Sometimes Drug Prices Are Too Low.” Wall Street Journal.
- Koons, C. (2018). “Generics Makers Need a Different Strategy.” Bloomberg Businessweek, New York: 15.
- Lee, S.L. (2019). “Quality Considerations for Continuous Manufacturing.” Food and Drug Administration, Center for Drug Evaluation and Research. Retrieved May 26, 2020 from https://www.fda.gov/
- Lee, S.L., O’Connor, T.F., X. Yang, C.N. Cruz, S. Chatterjee, R.D. Madurawe, C.M.V. Moore, L.X. Yu, & J. Woodcock. (2015). “Modernizing Pharmaceutical Manufacturing: from Batch to Continuous Production.” Journal of Pharmaceutical Innovation, 10(3), 191-199.
- Marsh, T. (2020). “The 20 Most Expensive Prescription Drugs in the U.S.A. GoodRx Blog. Retrieved May 26, 2020 from https://www.goodrx.com/
- McCarthy, Stephen. (2019). "Converting to a “Batch-Less” World: Quality Implications of Continuous Manufacturing." Retrieved May 26, 2020 from https://www.pharmaceuticalprocessingworld.com/
- Myerson, A. S., Krumme, M, Nasr,M, Thomas H, Braatz, R. D. (2014) Control Systems Engineering in Continuous Pharmaceutical Manufacturing May 20–21, 2014 Continuous Manufacturing Symposium. Journal of Pharmaceutical Sciences, 104, (3), 832 – 839
- NASDAQ OMX. (2020). “The Global Pharmaceutical Continuous Manufacturing Market was Valued at $393 Million in 2018.”
- National Conference of State Legislators. (2019) Generic Retail Drug Pricing and States 09/06. Retrieved May 25, 2020 from https://www.ncsl.org/
- Pagliarulo, N. (2018). “Pharma's slow embrace of continuous manufacturing.” BioPharma Dive. Retrieved May 26, 2020 from https://www.biopharmadive.com/
- Peter G. Peterson Foundation (2018). “How Will the Rising Cost of Prescription Drugs Affect Medicare?” Retrieved May 28, 2020 from https://www.pgpf.org/
- Price, E. (2018). “Continuous Manufacturing is the Wave of the Future.” PCI Synthesis. Retrieved June 17, 2020 from https://www.pcisynthesis.com/
- Ross, R. (2020). “Experts say COVID-19 will likely lead to US drug shortages.” Center for Infectious Disease Research & Policy News. Retrieved May 26, 2020 from https://www.cidrap.umn.edu/
- Sheikh, K. (2020). “Essential Drug Supplies for Virus Patients Are Running Low.” New York Times. Retrieved May 26, 2020 from https://www.nytimes.com/
- Snyder, M. (2016). The Benefits of Continuous Manufacturing. Pharmaceutical Processing World. Retrieved June 17, 2020 from https://www.pharmaceuticalprocessingworld.com/Toro, J.L. (2019). “Continuous Manufacturing and its Regulatory Challenge.” Lachman Consultants. Retrieved May 26, 2020 from https://www.lachmanconsultants.com/
- Witten, C.M. (2018). “CBER’s Perspective on Advanced Manufacturing.” Presented to ISCMP, London, England. Retrieved May 26, 2020 from https://issuu.com/