Case Reports: 2019 Vol: 25 Issue: 2
Megapharm: Production Efficiency or Customer Prioritization
Ricardo M. Pino, Pontifical Catholic University of Peru
Abstract
The author presents a study case that allows analyzing first, the pros and cons of two antagonist operations programming systems for make-to-order products: (a) FIFO with main customers’ prioritization; and (b) on a monthly basis, according of the orders received that month. Secondly, it allows the analysis of the difference between productivity improvement and competitiveness improvement. Finally, the case allows the analysis of the vertical integration between of the two main members of a supply chain: The producer and the distributor; although, in this particular case, the distributor owns the product brands and is also owns the producer company. The case allows the use of basic statistical tools, as Pareto analysis and forecasting. This case is to be used in supply chain management courses at MBA programs as well as in other post-graduate courses. It covers the topics of vertical integration and strategic decision making regarding a supply chain structure configuration.
Keywords
Competitiveness, Supply Chain Management, Productivity, Operations Programming System.
Case Synopsys
Megapharm is a manufacturing company in the pharmaceutical industry. It is one of the leaders, and face a high growth of its demand of its main product, pills; but at the same time, it face restrictions that prevent them from making additional investments in machinery or in new facilities. Carlos Zoer, general manager, urged Ramon Polo, chief of new products, to find a way to increase the plant capacity. The plant bottle-neck is the blister machine, that is used for almost all product lines, so only 20% of its time is dedicated to produce pills.
Megapharm is, besides, part of a supply chain with Europe Chemicals, its main customer and also the owner of Megapharm. In 1993, when Europe Chemicals bought Megapharm, 80% of its sales account for Europe Chemicals. At 2007, this percentage had dropped to 60% and it was projected that soon it would be 50%. Carlos Zoer has to decide how to increase Megapharm profits without compromise its relationship with Europe Chemicals. The analysis presents the challenge to figure out how to increase productivity and competitiveness without failing in the service level agreement with Europe Chemicals.
Introduction
On Tuesday February 14th, 2007, Carlos Zoer, General Manager of Megapharm, the largest pharmaceutical factory in Peru, called Ramon Polo, Product Development Officer, to his office: Ramon, I met yesterday with Giuliana Reyna and two supervisors of the pharmaceutical plant. I must admit that things have substantially improved when we changed the production lines schedule and we are already beginning to see the benefits. I still remember when overtime and corrective maintenance costs shot up, due to the increase of the demand during the first half of last year, and we had problems to meet deadlines in the line of pills. Now, this line complies 95% with the master plan. It was really a good job; however, I must tell you that it is not enough. There is still a 5% that is causing us trouble and I’m sure it will be hard to fix. Also, let me mention that I've met with important potential customers who would be interested in working with us in the long term, and this would represent substantial increase in the sales volume of pills; therefore, we must increase our production capacity. Ramon, we cannot afford to return to the previous stage. Giulianna’s Production Department believes that the solution to that 5% of trouble and the capacity increase lays in purchasing more equipment. They explained me their reasons, but before making this expensive investment we must be sure that it is really justified, because we have the cash allocated to projects that initiated earlier. What I’m asking you, given your vast experience in the area, is to study the line of pills in order to decide what to do to increase the production capacity in the short term, without expending money. Definitely, let’s avoid the entrance of an air bubble in the production tube. Giuliana will fully support you. I expect your report in a week’s time.
Brief History of Megapharm
Megapharm was born through a joint venture between Europe Chemicals (EC) and Indalab in 1993. The latter company owned Flexgrag pharmaceutical plant, which started operations in Peru in 1975, and whose assets became part of Megapharm facilities. Megapharm was a Peruvian company that provided services of processing and packaging pharmaceuticals, cosmetics, and natural products for both domestic and foreign companies. Megapharm produced solely for pharmaceutical companies.
In 1995, the pharmaceutical plant was remodeled to meet the standards of international Good Manufacturing Practices (GMP) and four years later, in 1999, it obtained the GMP certification. In the same year, EC bought the total shares of Megapharm and became the sole owner and main customer and it entrusted the manufacture of diverse products of their own lines and exclusive licences.
After the acquisition, Megapharm joined the Distribution and Logistics Services business unit of EC, to which Carlos Zoer began reporting to. Initially, Megapharm assisted only EC’s requirements, but eventually it began to take care of other customers’ needs. Carlos explained the emphasis put on quality:
“We are a company that cares about the environment, natural resources, and assists satisfactorily its clients; therefore, we have implemented the environmental management system based on the International Standard ISO 14001 since September 2001 and the quality management system based on ISO 9001 since December 2002, in 2016 we obtained the ISO 9001:2015 certification”
Appendix A shows the company milestones. By December 2016, it had roughly 550 employees and its annual revenue was USD 25 million.
Operations Management at Megapharm
Megapharm had two production plants: one for pharmaceuticals and the other for cosmetic products. Both plants were characterized by their flexibility to manufacture several pharmaceutical, cosmetic, and natural products. The plants were fully equipped with the latest technology and had a strict system of quality assurance. The autonomy to manufacture products, not only for EC but also for other customers, increased the company’s turnover. Thus, sales were gradually increasing, mostly in favor of other customers. They went from an 80-20 ratio in favor of EC in 2005, to 60-40 by the end of 2006, and projected to be 40-60 in 2008. For instance, in the line of pills, the most important products were Calmatex, Vient 40, and Faranat sugar-coated tablets and only the latter of these was produced for EC.
Megapharm also provided product design and development services to its customers. Hence, it had a department that studied, evaluated, and developed new products or reformulated old ones, as needed. This department was under the supervision of the Technical Director’s Office.
The Pharmaceutical Plant and the Line of Pills
The pharmaceutical plant revenue was around USD 20 million a year. This plant met the needs of the local market and manufactured about 350 products in 700 presentations, which were grouped into 33 product lines, from which 16 were for international customers. Manufacturing operations were oriented to intermittent processes (batch). In order to achieve efficient processes that allow making these products on time and without incurring in extra costs, the company established four production processes: solids, semi-solids, packaging, and liquids. The processes of solid and semi-solids are subdivided into production lines, as shown in Table 1.
| Table 1 Production Lines For Each Production Process | |
| Production Lines | Production Process |
| Powder | Solid |
| Pills (coated and non-coated) | Solid |
| Capsules | Solid |
| Creams (creams, ointment, unguent, and gels) | Semi-solid |
| Vaginal pessaries and suppositories | Semi-solid |
| Conditioning | Packaging |
| Liquids (syrup, suspensions, and drops) | Liquid |
The seven lines shared both equipment and labour. Many machines were used for more than one production line, which made it difficult to calculate the plant capacity. For example, the line of pills had a maximum monthly capacity of 21 million units, which would be achieved if this line used the equipment exclusively. However, as it shared some equipment with the lines of capsules, powders and conditioning, the actual production in this line was less than 20% of its maximum value.
On the second half of 2006 the way to schedule product manufacturing in the line of pills was changed, thus improving the production plant capacity. Many of these changes were made possible following recommendations prepared by Ramon. He stated, among other things, that as the Gamma sealing machine for blister packs was the bottleneck in the line of pills, in order to determine the products manufacturing sequence it should be considered the similarity between the products rather than the order in which the customer orders were received, so as to reduce machine setup times. Also, preventive maintenance should not only consider machines lubrication, but all machine needs, since in this way, equipment availability was increased. Ramon felt that the general manager trusted him as a result of his analysis and he was not mistaken. Carlos liked Ramon’s alertness, since he had already intuited that any proposal to improve the performance of the line of pills would significantly impact revenues of the pharmaceutical plant.
From 2005 onwards the line of pills earned the most part of the pharmaceutical plant revenue. It contributed in 2006 to 35% of total revenue, USD 8.8 million. In addition, Megapharm had set as a goal to develop 18 new products for this line in three years, which would represent 40% of all the strategic products to be developed in the seven lines. Productivity of the line of pills was measured by indicators that related monthly revenue with cost of resources used that month-whether they were labour (man-hour productivity) or equipment (machine-hour productivity).
Manufacturing and Packaging
The pharmaceutical production plant was divided into two separate but physically contiguous areas: Pharma Manufacturing and Pharma Packaging. Giuliana, the Production Manager, was in charge of both of them. Each area had a supervisor and the work of the seven lines were executed in them both. To begin the manufacturing process, the supplies or raw material were requested to the warehouse supervisor, who was responsible for authorizing the entry of raw material into the plant from the bulk warehouse.
The production plant complied with GMP principles to ensure a high level of purity when manufacturing the products. For this, two main areas were clearly delimited: the gray one and the black one, regarding the required level of cleanliness in the environment. Both Pharma Manufacturing and Pharma Packaging areas had a high level of hygiene, so that they were classified as gray areas.
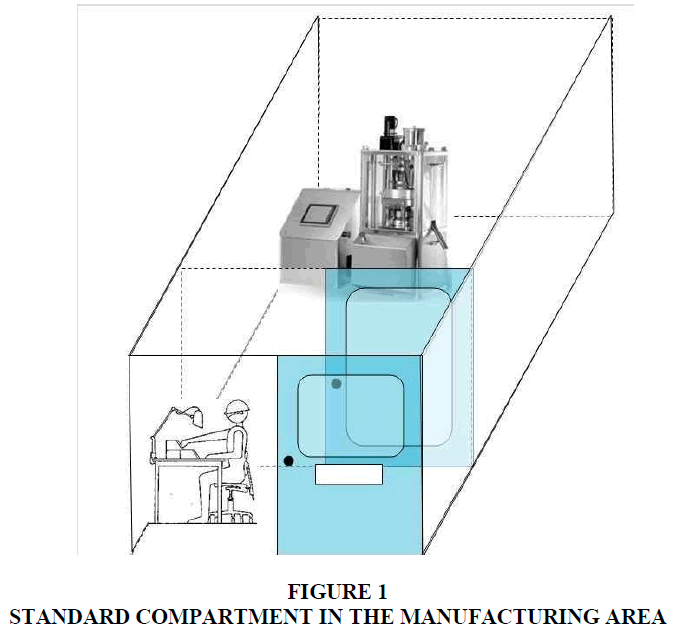
All activities related to product development were made in the Pharma Manufacturing area. This area was 1.175 m2, one floor. The machines were expensive and specialized, and they were located within compartments (Figure 1), which were environments with positive pressure and filtered air. Some of the compartments had a single machine while others had several similar machines but with different capacity. According to GMP, the use of each compartment was restricted to the manufacture of one product at a time. This meant that if not all the machines in a compartment were used for the manufacture of a batch, the remaining ones could not be used in the manufacture of another product during the production of that batch.
The machines from the solid-products lines were located in consecutive compartments on one side of the long corridor of the manufacturing plant. The first was the granulation and drying compartment, followed by two granulation compartments, one for powder blending, three for compression, and one for coating or sugar coating. Also, many compartments had gates or preceding rooms equipped with a table and a chair, where the operator registered the performed activities. These compartments had a single entrance to carry the products in and out. There were two types of containers used to move the in-process product to other compartments: In the case of solids, plastic drums with a white lid were used and metallic and air-tight cylinders were available for liquids and semi-solid products.
The pharma packaging area had a surface of 472 m2. All primary packaging (blisters or tapes) activities were executed in this area, as well as labeling, packing, and boxing up. In the case of pills, the primary product container could be made either in the Gamma sealing machine or the Uhlman wrapper. The sealing machine for blister packs was the most used, since the quantity of products that passed through had a 9:1 ratio in regard to the wrapper. These machines were located in compartments that had an entrance area to receive the products to be packaged and a separate exit connected with the packaging tables. The operators were working in those tables, receiving the packaged products in their primary container for the subsequent packing and boxing up
The Line of Pills Productive Process
The objective of this line was to convert raw materials into high quality pills and sugar- coated tablets. For this, there were several sub-processes: Granulation, drying, powder blending, compression, coating or sugar-coating, and primary packaging. Not all pills passed through all the sub processes. For instance, some pills started the manufacturing process with powder blending; they didn’t pass through granulation because they had moisture sensitive compounds (Appendix B shows the list of machines used in each sub-process in the line of pills).
Granulation
This sub-process consisted in kneading the blend with a liquid (water and/or alcohol) in order to increase the particle size and facilitate the compression. In this sub- process, the product could pass through three machines. Depending on the product type, it could go either one of the two Molteni or the Diosna. Whether a product goes in one Molteni or the other would depend on the batch size to be manufactured.
Drying
In this sub-process, the granules were exposed to temperatures ranging from 35°C and 80 °C (depending on the type of ingredients) in order to remove the excess of moisture. There were four stoves and a Glatt dryer for this. The latter was used for products different from the ones on the stoves. Stoves II and III were in the same compartment and their use depended on the batch size.
Powder blending After the drying sub-process, it was necessary to make a homogeneous mix. However, the powder that didn’t require granulation was directly blended. There were two blenders that were used depending on the batch size to be processed.
Compression
This sub-process consisted in compacting the powders or granules according to the defined doses and weights of the tablets. This sub-process had six machines distributed in three compartments. These machines were exclusive, because they had different types of punches that worked on different products. Manesty B3B, Stokes B2 and Killian machines were quite old. The Riva C3 tableting machine was new and had been purchased to replace the previous three, since it had the available capacity to take on the production of the oldest. This tableting machine was located on the second compression compartment. Riva C3 needed to be adapted in order to replace the old machines, for which it was necessary to buy three sets of punches and matrices valued at USD 3,000 each. As for the other machines, the Korchs was responsible of only one product, which was already discontinued, and the Manesty D4-located on the first compression compartment-took mostly the products that other tableting machines could not take on.
Coating
Some products coming from the sub-compression process needed a prior coating (sugar-coating) before being sent to the primary packaging sub-process. For this, drums I, II and III could be used as they were arranged in a single room or the mobile drum IV that was located separately. The drums that shared room were chosen according to the batch size to be manufactured.
Primary packaging
The tablets coming from compression and the sugar-coated tablets that came out from the coating sub-process went to the primary packaging sub-process. Depending on the product, they could go into the Gamma sealing machine for blister packs or into the Uhlman wrapper. The first one was, according to Ramon, the bottleneck of the line of pills, as it was the only sealing machine for many products, the same that was responsible for approximately USD 5 million of annual revenue.
Planning and Scheduling
The planning was developed annually and reviewed monthly. For EC’s orders, its logistics planners-several of which used to work at MEGAPHARM logistics área-were responsible for production planning. Hence, an annual master production schedule (MPS) was developed, which consisted in a sequence of two fixed months and the rest adjustable. EC sent MEGAPHARM the MPS and the production orders by the end of each month. As for the products for third parties, i.e., those which were not for EC, MEGAPHARM staff received from their customers a forecast for the following months. Planning consisted in determining the products and quantities that must be produced every month, adding up the different requirements received from third parties. MEGAPHARM used the BPCS system for the bill of materials explosion of EC and third parties’ products.
Before implementing the improvements in the production lines, each time a purchase order from third parties or a production order from EC was received, Giuliana, along with the two Supervisors of the pharmaceutical plant and the warehouse Supervisor, scheduled the production for the received order. Back then, customers placed their purchase orders any day of the month; the schedule was made mainly on a first-come-first-served basis and the production started as soon as the machines were available. When an important customer sent his purchase order with an urgent request, this had priority over other clients that were less important for the company. In this scenario, EC’ orders always had priority over third parties’ orders. This way of managing requirements had caused to reschedule activities several times in order to fulfill the orders of the most important customers. As a consequence, in more than one occasion and despite the overtime, deadlines for third parties’ orders were not met. This led to an efficient customer service for the most important clients; nevertheless it caused an inefficient productive process.
After implementing changes in the second half of 2006, the schedule was developed monthly and per production line. Third parties and EC’ orders were received in compliance with a new policy, for which the customers sent their manufacturing orders at the end of the month and they were processed within thirty days. The production began on the first working day of the following month. If a client sent his order late, the original schedule wasn’t modified. Giuliana explained:
“If an order was received late and it could still be added-up to the schedule, then we gave a delivery date, but if it was not added-up due to a lack of capacity, the customer was notified and he/she could choose whether to wait an extra month or to withdraw the order. Now, the schedule is developed according to the plant capacity and not in relation to the date set by the customers. Our delivery time is one month once the production process start, although sometimes it takes more time. Therefore, in the future we will try to receive the orders on the 20th day of each month instead of waiting for the last day, which would let us do a better monthly schedule... Another important thing is that we coordinate better with EC logistics department and the number of rush orders have decreased”.
According to this new approach, at the end of the month a group of orders were classified in order to obtain the total quantity that each line will produce, i.e., “the products that will go through the same machines”. The line of pills, within the solids production process, was always the most congested which is why it was scheduled first. “The three supervisors and I coordinated and developed the monthly schedule in Excel and it took us about five hours. This schedule detailed the line, machine, and the responsible operator assigned for each day” stated Giuliana. “In addition, we also consider preventive maintenance time when scheduling, because those guys responsible for maintenance and quality control participate in the meetings”. This approach allowed the production activities not to be isolated from these areas, as they had been some months ago. However, the majority of unexpected events that still occurred in the execution of the production schedule were caused by machines defects or in packing material.
The production program was developed as of the first sub-process. If the original delivery date was extended it was returned from the last machine in the packaging sub-process-mostly to the sealing machine for blister packs-and the planned activities for the machines of the prior sub-processes were re-scheduled in order to comply with the agreed commitment.
Setup and Cleaning
Two things were important to consider whenever the use of a machine was scheduled. The first was the setup time, in which the machine was arranged for the production of a certain type of product. Each type of product had a specific manufacturing format with an identification number. The new way to schedule allowed grouping products under similar manufacturing formats, thus reducing machine setup time. For example, in the case of the Gamma sealing machine for blister packs, large quantities were accumulated by format, since die-change time could be two to three hours.
The second thing was the machines’ cleaning. It should be carried out in depth each time the production of a batch was finished. The products, that due to its composition left the machines dirty or stained, were manufactured on machines that didn’t share room. For example, in the coating compartment drums I, II, and III were in the same room, but drum IV was mobile and it was used only for “very dirty” products, otherwise the cleaning time of the room would delay production schedule.
Maintenance and Quality Control
Similarly, with the new scheduling system, preventive maintenance operations were scheduled at the start and at the end of each month; since those were the days the production plant was not fully in use. The maintenance assistant developed the maintenance program one month in advance, according to the production schedule, in order not to interrupt the production process. “Now we are more aware of the importance of preventive maintenance and we work with them coordinately. We are no longer reluctant to accept their plan” said Giuliana. A preventive maintenance plan wasn’t done before in the plant, mainly because of the high workload. The inspections and lubrication carried out to the machines tried to replace the preventive maintenance tasks. This, obviously, was not enough, since some critical machines were damaged and this led to stops that caused significant expenses to the company.
For instance, Gamma sealing machine registered in 2016 five monthly repairs. Each repair caused a 4-hour corrective maintenance work. This machine operated an average of 164 hours per month (excluding setup times) and if a serious failure occurred, it would have taken approximately 30 days to be repaired, as the manufacturer of these machines had ceased operations and it was not easy to get original spare parts. The cost of a new sealing machine for blister packs was around USD 380,000.
The second most important machine for the line of pills was the Manesty D4, which was located in the first compression compartment. In this machine, 33 products of the line of pills were produced, and last year there had been an average of 10-hours stops for corrective maintenance per month due to high workload and the lack of a preventive maintenance plan. In general, it took about 8 hours to the machines of this area to return to their operating status after suffering a malfunction.
Quality control was carried out throughout the production process, especially at the end when the approval of the finished product was granted and delivery of the product to the client was authorized. Quality control was also carried out at the end of each sub-process. The quality control personnel went to the compartments, inspected and gave their approval or disapproval, but sometimes it was not right away because the personnel was not available when needed. This prolonged the time cycle, and the workers knew it was not possible to proceed to the next sub- process without the approval of the quality control department. Moreover, the operator of the subsequent sub process didn’t accept the products of the previous sub-process without the signature of the quality control manager.
With regard to the events due to poor quality in the line of pills, these accounted for 32% of all events produced in the seven lines. These events included the batches of rejected or reprocessed products, as well as complaints from the customers. For Carlos, the quality control area was a black box, “We can’t measure time; all the machines are strange... We need to understand this function, if we don’t we can be wasting money where we shouldn’t”.
Supplies and Packing Material
The supplies used in the manufacture of the production orders submitted by EC were provided by it and stored in Megapharm’s warehouse. Ramon explained: “EC buys the supplies of the products requested by them. These come directly to our warehouse. With the printed logistics order along with the list of materials sent to us, they allow us to use that inventory”.
In Megapharm the supplies and packaging materials-which were also provided by EC-were analyzed. In case of not having the necessary supplies for the manufacture, the schedule was not developed. This was similar in the case of third parties’ requirements, mostly because they brought their supplies too.
Staff
Two shifts were established in this plant. The first shift was from 7:30 h to 17:15 h and the second shift was from 19:30 h to 7:30 h of the next day, both with a lunch break of 45 minutes. Total productive hours per day in both shifts was about 20 and the working days per month were 22, on average. Workers received a fixed salary for their work.
The available man-hours were distributed among the lines. There wasn’t an exclusive staff for each of these and many operators knew how to operate several machines. When manufacturing operators hadn’t a lot of workload, they helped in the packaging area, but it wasn’t the other way around, since the personnel operating the machines in the packaging area had lower skills. However, in the manufacturing area not all operators were highly skilled. For example, there was an expert and three support operators moderately trained to operate the sealing machine. A three-month training course in the use of the Gamma sealing machine for the three support operators would cost USD 3,000.
In general, labour was trained but the training course considered only theory and not practice, especially about GMP and safety. There weren’t specialized training courses about the mahines operation yet because the manufacturing personnel didn’t rotate much and they were experienced workers.
The Orders Dilemma
It had been two days since Ramon left Carlos’ office thinking about how to improve the productive capacity of the line of pills. In those two days, he had talked to Giuliana about the orders, he had spent long hours in the plant and he already had enough information to start his analysis.
On Thursday 15th at night, while walking toward the parking lot, an idea suddenly crossed Ramon’s mind, something that he couldn’t even imagined moments earlier. He realized that, in reality, there were two opposing paths to make an analysis and suggest solutions: The first and most obvious was conservative: he would have to propose improvements to the current system, and the other definitely more radical and riskywas opposed to the first one and it might kill him.
At home, as he considered the two possible options, he drank a cup of hot dark coffee that his wife had made him. The coffee tasted bitter despite of the amount of sugar that she put in it. They had plans to go out this weekend, but Ramon was too distracted on resolving the dilemma. He thought:
“Things are working now. Yes, we have improved a lot and we are all aware of that, but I’m afraid that we may not get much more capacity if we keep the policy for orders receipt and schedule, or even worse, if the demand of pills increases this year and causes a snowball again... perhaps we should consider returning to the old system of receiving orders, but fixing the errors that caused us so much harm”.
Suddenly, a new issue came to his mind: What would the position of EC be if Megapharm continues increasing the use of its limited plants capacity to attend other customers’ needs instead of theirs? Wouldn’t EC expectation be to receive priority attention? Ramon realized he had to take into consideration not only operative measures, regarding production efficiency and shortening delivery times, but also strategic consideration, as how to increase revenues without forgetting EC expectations as MEGAPHARM still main customer. He would not forget, either, that his recommendations should not require machines purchasing. Ramon took the last sip of his dark coffee, took his laptop and went to the desk. Tonight he should start writing the report if he wanted to finish on time.
Appendix
| Appendix A Important Milestones | |
| 1975 | FLEXGRAG (INDALAB) plant construction |
| 1993 | Joint Venture (50% INDALAB, 50% EC) |
| 1999 | GMP/GMP certificate for the pharmaceutical plant (issued by DIGEMID, Department of Health) |
| 1999 | Purchase of 100% of the shares by EC |
| 2000 | GMP/GMP certificate for the cosmetic plant (issued by DIGEMID, Department of health) |
| 2001 | ISO 14001:2002 certificate |
| 2002 | ISO 9001:2000 certificate |
| Appendix B Sub-processes equipment of the line of pills and use frequency | |
| Equipment | Frequency of use (times per week) |
| Granulation | |
| Molteni PH-300 (300L) | 5 |
| Molteni I-150 (150 L) | 6 |
| Diosna (150 L) | 2 |
| Drying | |
| Heater I | 5 |
| Heater II y III | 10 |
| Heater IV | 1 |
| Glatt | 3 |
| Powder blending | |
| Hoop mixer (200 L) | 13 |
| V mixer (600 L) | 5 |
| Compression | |
| Manesty D4 (30.000 c/h) | 11 |
| Manesty B3B (25.000 c/h) | 1 |
| Stokes B2 (25.000 c/h) | 0 |
| Riva C3 (75.000 c/h) | 3 |
| Killian (30.000 c/h) | 0 |
| Korchs (10.000 c/h) | 1 |
| Coating | |
| Bombo I (60Kg), II (60 Kg), and III (80 kg) | 6 |
| Bombo IV (60 Kg) | 0 |
| Packaging | |
| Gamma blister (7.000 b/h) | 16 |
| Uhlman tape machine | 1 |
A Pareto diagram was developed to obtain the frequency for 10 products of the line of pills.
Teaching Notes
Teaching Objectives
1. To understand how strategic decisions of an organization can affect the relationship with the other supply chain members.
2. To comprehend how decisions that affect competitiveness of organizations could have a higher impact that those that affect productivity.
3. To analyze pros and cons of the operations programming system FIFO against production programming on a monthly basis.
4. To define strategies for a supply chain configuration, learning that what is best for a member is not always better for the supply chain members as a whole.
Discussion Questions and Suggested Answers
The initial part of the discussion should establishes the kind of organization that Megpharm is: For one side, Megapharm do not purchase raw material as their customers provide it. Its profits depend on the efficiency by which they execute the services required by their customers. For the other side, the very first reason of their existence is to allow Europe Chemicals to have in-house production for its own products. The growth history of Megapharm is relevant too, as the current situation seems not to be the initial one, at least in the relationship with Europe Chemicals. These days, Megapharm has gained a market share that is growing, mainly for the increasing sales of their most important product: pills.
1. Why did Carlos Zoer trust Ramon Polo to improve the production plant capacity?
The reason is that in the past, Ramon proposed the change of an operations programming system, FIFO plus prioritization of customers, which by large favors Europe Chemicals, to one in which all supply orders are received and registered, but not produced, until the monthly production program is developed. That change allows passing from a system that favored the attention to certain customers (Europe Chemicals in this case) to one in that the efficient use of the plant resources was prioritized. As the changed proved effectively, Carlos was grateful to Ramon.
2. What advantages and disadvantages do you appreciate in the initial operations programming system regarding the new system used by Megapharm?
Regarding the advantages of the initial system, programing operations in the same order as customer orders arrive, plus prioritization of customers, many Banks and Airlines used this same system (Vilcu et al., 2018). Those organizations classify their customers by their degree of importance for the company and allocate resources to serve them accordingly. The old system was efficacy oriented and was customer focused, at least the bigger ones. The main problem was that it generated inefficiency in the use of many resources, as machines that remain unutilized, as not all customers require the same products.
In the new system, all customers are attended with the same priority, and the system is regulated through efficiency in the use of resources. Although this guaranties a better attention to customers as a whole, the ones that were treated with priority in the old system would be affected with the new one. In other words, if in the macro level the new system looks good, at a micro level it could be originated that at least few customers could go away if they do not receive the same service level they were used to.
3. What steps do you consider that Ramon could suggest Carlos to increase the production plant capacity? You have to take into consideration that the company is not able to expend money in investments.
Regarding the steps that Ramon would recommend to increase the plant capacity, as he is chief of new products, it is expected that he make proposals that improve the use of resources, which is to increase productivity (Ahumada & Cuevas 2010). These actions could be:
1. To pass from two working shifts, of 10 hours per day each, to three shifts of 8 hours each. Although this could generate complains, as working hours would be reduced, when they increase night working hours they would receive additional compensation. It is not an objective of the case to analyze how to negotiate with workers, but to show that a fixed asset not plenty used represents an opportunity for the organization.
2. To coordinate better the quality control activities. As the production process is structured in lots and to assure process quality, when a production step finishes, the quality control department has to evaluate the product-in-process pipeline and give the conformity before the production process could continue with the next step. Control quality department could cause delay in the production if they do not act promptly when a step of the product is finished, so the communication between production and quality control becomes extremely necessary.
3. In-house personal training. To have personnel that could perform in more than one position would allow them to diminish the risk of not producing on time if the specialized workers are not available. In-house training increases productivity and flexibility in organizations.
4. Put each machine in a different cubicle. It will be necessary to adapt the plant layout to separate machines. This will allow the plant to operate at maximum capacity when required. In the actual system when a machine starts, the machines not used in the same cubicle cannot be used until the first machine is turned down.
5. To improve predictive maintenance and operations programming, to have a better availability of the production machines and a better efficiency in the use of resources.
4. What steps could Carlos Zoer take to improve competitiveness at Megapharm? How are those actions different to the ones that Ramon would propose?
Regarding steps to increase competitiveness, remember that competitiveness is defined as the capacity to compete successfully in the market. It is to say, competitiveness is the capacity to offer valuable products to customers, which give the company a superior return as the one obtained by its competitors. Competitiveness must generate profitability for the company. To Megapharm, the most profitable product is pills. However, only 20% of the available time of the blister is dedicated to the pills production. What this means is that 80% of its time is dedicated to the production of products that are not so profitable. Besides, the meeting that Carlos had with potential customers was because they are interested in buying pills. Because of these reasons, Megapharm could redefine its products mix to give more room for pills production. Remember that, being a company that receive inputs from its customers, Megapharm only produces on a make-to-order basis, not having inventory of raw material or finished products. As so the steps that Carlos could take could be:
1. To increase the price of all products but pills. Although the increase percentage would not be discussed, as the case did not bring cost information, it is to expect that a price increase would have positive effects for Megapharm. For one side, profitability of these products will increase, making their sale more attractive for salespersons. For the other side, the sold quantity will diminish, making room for the blister to make more pills. This could happen if customers who buy pills buy the other products as well. Although it is possible that this happens, it is unlikely because, at least, potential customers are interested mainly in pills.
2. To decrease sales commission for all products but pills. Although many students could argue that to decrease commission could generate dissatisfaction and demotivation in salespersons, this is arguable, as when it became not so convenient for them to sale those products, they will assign more effort to sale pills, that is exactly the product that are more interesting for Megapharm to sale. Many students could propose that it would be better to increase the commission for pills sales, instead of decreasing commission for the other products, but that is arguable as increasing commissions for selling products whose demand is increasing is not the best decision under managerial viewpoint. For the other side, the less commission for the other products will be compensated with the increased sales of pills that each salespeople could make.
5. If you were Europe Chemicals CEO, What production programming system would you prefer for Megapharm? In the former system, Europe Chemicals was the prioritized customer, although in the new system it is treated as if it were one of the other customers.
Finally, from the Europe Chemicals point of view, Megapharm decisions to expand through the increase on pills sales to other customers, and to not prioritize the attention to few customers, but to use more efficiently its infrastructure, could affect the relationship between both companies. If this becomes the situation, Europe Chemicals leaders could react in one of two ways: (a) To refrain Megapharm activities with its other customers, and in that way continuing being its main customer, which will keep the relationship as it is now and give support to Megapharm to expand without compromising the relationship between them both, or (b) To leave Megapharm to expand as they consider the best way for them.
To analyze pros and cons of every possible decision, it has to be taken into consideration that the main reason why Europe Chemicals acquired Megapharm was that it required a production plant for their products. It is in this regard that, at the beginning of the relationship, 80% of the Megapharm production was sold to Europe Chemicals. However, through the years this situation changed and at the actual moment this percentage is 60% and it is expected than in the future it will be 50%. This shows that, either Megapharm has been increasing its plant capacity or Europe Chemicals has been steadily decreasing its purchases to Megapharm, maybe because they are decreasing its market share or because they have found other suppliers for their products, the latter being more plausible. Regarding these two possible decisions:
1. If Europe Chemicals opt for restraining the expansion of Megapharm, establishing that they will continue as priority number one, sooner or later Megapharm customers will find other supplier that bring them a better service. As so, it is expected, then, that while Europe Chemicals assures the supply of products to sell, it also favor the emergeing and growth of Megapharm competitors. For the other side, Megapharm do not obtain economy of scale nor could develop its competitiveness focusing on the products they have competitive advantage to make. In the long run, it is expected to be beat by its competitors, a thing that will put in risk also Europe Chemicals, as several Megapharm competitors will attend Europe Chemicals competitors also.
2. If Europe Chemicals opts for leaving Megapharm free, then the latter could increase their growth and make more profits. However, Europe Chemicals could have troubles at not receiving a personalized attention. Those problems could be resolved going back to the initial situation and acquiring a new company that replace Megapharm. This new company would be the size that Europe Chemicals need. The other possible solution is to improve their planning process in order to make the attention that they receive from Megapharm good enough for them (Lima & Batagila, 2018).
| Teaching Plan | Time (Minutes) |
|---|---|
| Outline main Megapharm activities | 10 m |
| Analysis of both operations programming system | 10 m |
| Analysis of actions to increase productivity | 15 m |
| Analysis of actions to increase competitiveness | 15 m |
| Customer-supplier relationship in supply chains | 20 m |
| Conclusions | 10 m |
| Total | 80 m |
References
- Ahumada, V. & Cuevas, M. (2010). International competitiveness, productivity and unit labor costs in the manufacturing Industry. Frontera Norte. jul-dic 2010, 22(44), 7-39.
- Vilcu, A., Verzea, I., & Herghiligiu, I. V. (2018). Challenges and innovation in management and entrepreneurship. Social and Behavioral Sciences, 238, 424-431.
- Lima, A. C., & Batagila, W. (2018). How diversification affects vertical integration through experience in pharmaceuticals. Journal of Technology Management & Innovation, 13(4), 3-12.