Research Article: 2022 Vol: 25 Issue: 6
A Deep Learning Based Clinical Decision Support System For Malaria Diagnosis and Detection
Alamu Femi O, University of Lagos
Abiodun Adeyinka O, Bowen University
Jinadu Ahmad Adekunle,University of Lagos
Citation Information: Femi O, A., Adeyinka O, A., & Adekunle, J.,A. (2022). A deep learning based clinical decision support system for malaria diagnosis and detection. Journal of Management Information and Decision Sciences, 25(6), 1-9.
Abstract
Malaria remains one of the major challenges faced in healthcare in Africa, especially in Nigeria with an estimated 300,000 children killed by malaria annually. Apart from low doctor-to-patient ratio in Nigeria, poor diagnosis is another major cause of increase in malaria death rate.
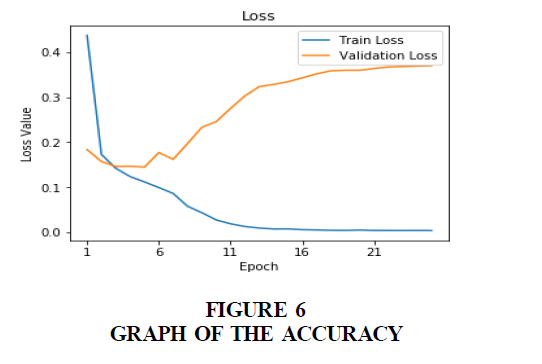
This research developed a Clinical Decision Support System (CDSS) to detect malaria-infected patients using Deep and Machine learning technique. For this, we developed an in-depth learning method from camera captured Giemsa-stained thin blood smear slides from 150 Plasmodium Falciparum-infected and 50 non-infected patients from a National Center for Biomedical Communications. The dataset contains 27,558 cell images with equal number of malaria-infected and non-infected cell images which are 13,779. The architecture of the proposed model predicted patient’s malaria status and was evaluated using 5-fold cross-validation. The images were preprocessed and resized after which the learning stage began. Deep learning and classification were carried out using Convolutional Neural Network (CNN). The CNN model was trained by using stochastic gradient descent (SGD) and Nesterov’s momentum to optimizing the multinomial logistic regression objective. The proposed model achieved a training accuracy of 99%, validation accuracy of 97%, 40% train loss, 35% validation loss and 98% prediction.
Keywords
Malaria, Deep learning, Machine Learning, CDSS, CNN.
Introduction
In recent years, there has been a substantial resurgence in the optimistic disposition in the use of artificial intelligence tools and techniques in healthcare. This revitalized optimism originates, at least in part, from the recent advancement and achievement in artificial intelligence techniques such as machine learning and deep learning research in the non-healthcare sector of the industry. The innovation and construct of various machine learning and deep learning algorithms to tackle health and other challenges have existed for some time, but the enhanced availability of the massive amount of data, matched with equally impressively powerful computers, enabled these achievements and breakthroughs seen in machine learning and deep learning this decade (Dipanja, 2019).
In medical domain diagnostic, classification and treatment are the main task for a physician. System development with such purposes is also a popular area in Artificial Intelligence (AI) research. Computer-Aided System or Decision Support System (DSS) that can simulate expert human reasoning or serve as an assistant of a physician in the medical domain is increasingly important. Today, clinical Decision Support System (DSS) are developed to act multi-purposed and are combined with more than one AI method and technique (Blobel et al., 2014).
In Nigeria, there are several challenging health problems that need addressing utilizing public health rules in order to improve stability. Over the years, there have been some programs initiated to deal with these healthcare challenges in Nigeria but they have all brought about little or no betterment in our healthcare condition. In addition, the persistent disregard of the necessity of dealing with public healthcare challenges would worsen matters for the majority of the Nigerian population who are directly impacted by these issues. A report on non-communicable diseases by the World Health Organization stated that Nigeria and other developing countries are the worst impacted with a high mortality rate from non-communicable diseases. Based on statistics released by the World Health Organization in 2017, the mortality rate of people infected by malaria in Nigeria is 146 per 100,000 populations (Bolaji, 2016).
With malaria being the most prominent cause of death in Nigeria. It has the greatest effect of disease in the country (Bolaji, 2016). With an estimated 300,000 children killed by malaria annually. It causes more than 25% of infant deaths, 30% of childhood deaths, 11% of maternal deaths. Moreover, there are at least 50% of Nigerians with at least one encounter with malaria annually, at the same time, children below 5 years of age have around 2 to 4 encounters yearly. Malaria is predominantly severe amongst children below 5 years of age and pregnant women because of relatively weaker levels of immunity. Classification of these parasites was carried out by (Abiodun et al., 2021) with P. Falciparum the most predominant malaria parasite in Nigeria and subsequently the cause of most malaria-associated deaths. Other malaria parasites such as P. vivax and P. Knowlesi can also cause severe malaria and death but they put up significantly less effect than P. Falciparum. Wrong diagnosis will lead to wrong treatment and eventually lead to more mortality coupled with the fact that the need for experts far outmatches the available supply in the developing nations and sometimes physician performance is mostly not straight-forward because of diagnostic mistakes due to Inefficient incorporation and collaboration of health information technologies (Health IT), Gaps in communication among clinicians, patients, and their families as well as a healthcare work system which, by design, does not sufficiently support the diagnostic procedures. There is therefore a need is to implement a deep learning-based clinical decision support system that is capable of recognizing patterns in malaria patient symptoms to make a possible diagnosis so as to assist and hasten a suitable course of action (Schroff et al., 2015).
Literature Review
Computer technology approach to diagnose different diseases is an ongoing research in Information Technology and health domain. Many clinical decision support systems are a two-layer knowledge base model of rule reasoning and they do not express knowledge very well since it simply infers disease from the presence of certain symptoms. (Jiang et al., 2017) proposed a three-layer knowledge base model (disease-symptom-property) to utilize more useful information in inference. The system iteratively calculates the probability of patients who may suffer from diseases based on a multi-symptom naive Bayes algorithm, in which the specificity of these disease symptoms is weighted by the estimation of the degree of contribution to diagnose of the disease and found that the three-layer model can improve the accuracy of predictions compared with the two-layer model and highlighted several areas that need continued improvement.
Machine learning vows to upset clinical decision making and diagnosis. In medical diagnosis a doctor expects to clarify a patient's manifestations by deciding the sicknesses causing them. However, existing machine learning approaches to diagnosis are purely associative, identifying diseases that are strongly correlated with a patient’s symptoms, (Richens et al., 2020) reformulates diagnosis as a counterfactual inference task and derive counterfactual diagnostic algorithms comparing their developed counterfactual algorithms to the standard associative algorithm showing that causal reasoning is a vital missing ingredient for applying machine learning to medical diagnosis (Teoh & Kidd, 2017).
A systematic review to investigate the association between the interactive use of ML-based diagnostic CDSSs and clinician performance to inspect the degree of the CDSSs' human variables assessment utilizing MEDLINE, Embase, PsycINFO, and dark writing between January 1, 2010, and May 31, 2019 was led by (Vasey et al., 2021). A sum of 8112 examinations was at first recovered and 5154 modified works were screened; of these, 37 investigations met the incorporation models. The middle number of taking part clinicians was 4 (interquartile range, 3-8). Of the 107 outcomes that detailed factual importance, 54 (half) were expanded by the utilization of CDSSs, 4 (4%) were diminished, and 49 (46%) showed no change or an indistinct change. Expressing that more exhaustive assessment ought to be directed prior to conveying ML-based CDSSs in clinical based CDSSs in clinical settings with (Heyen & Salloch, 2021) adding to the moral conversation by utilizing professionalization hypothesis as an insightful focal point for examining how clinical activity at the miniature level and the doctor- patient relationship may be impacted by the work of ML_CDSS. Presuming that the more routinized the utilization of ML_CDSS becomes in clinical practice, the more that doctors need to zero in on the patient's anxiety and fortify patient independence, for example, by enough incorporating digital decision support in shared decision-making (Danyliv et al., 2013).
Machine learning (ML) methods were used on blood tests data to predict COVID-19 mortality risk (Karthikeyan et al., 2021). A powerful combination of five features: neutrophils, lymphocytes, Lactate Dehydrogenase (LDH), high-sensitivity C-reactive protein (hs-CRP), and age helps to predict mortality with 96% accuracy. Various ML models (neural networks, logistic regression, XGBoost, random forests, SVM, and decision trees) have been trained and performance compared to determine the model that achieves consistently high accuracy across the days that span the disease. The best performing technique utilizing XGBoost highlight significance and neural organization arrangement, predicts with a precision of 90% as right on time as 16 days before the result. Hearty testing with three cases dependent on days to result affirms the solid prescient exhibition and reasonableness of the proposed model. Performing nitty gritty examination and recognizable proof of patterns utilizing these critical biomarkers to give valuable bits of knowledge to natural application while (Choi et al., 2020) developed a machine learning-based Clinical Decision Support System (CDSS) for recommending initial treatment option in HCC and predicting Overall Survival (OS). The six multi-step classifier model was developed for treatment decision in a hierarchical manner, and showed good performance with 81.0% of accuracy for Radio Frequency Ablation (RFA) or resection versus not, 88.4% for RFA versus resection, and 76.8% for TACE or not.
Advancements in Deep Learning (DL) for healthcare cannot be left out and are tremendous in the recent years because of the way that there is accessible and immense measure of information for investigation. A wide assortment of DL algorithm is being utilized and being additionally evolved to tackle various issues in the medical healthcare environment. Clinical medical care is one of the premier regions in which learning algorithms have been attempted to help navigation. Such sort of wise decision making in medical services and clinical practice is likewise expected to bring about comprehensive therapy. A section on headings for future exploration like dealing with class lopsidedness in analytical information, DL for guess triggering preventive consideration, information protection and security were checked on and collected. Different extant DL procedures and their applications for choice help in clinical frameworks using fundamentally three application types of DL, namely image analysis, natural language processing, and wearable (Sandeep Kumar & Jayadev, 2020).
Qjidaa et al. (2020) proposed a clinical decision support system for the early detection of COVID 19 using deep learning based on chest radiographic images. An in-depth learning method which could extract the graphical characteristics of COVID-19 in order to provide a clinical diagnosis before the test of the pathogen was developed. 100 images of cases of COVID-19 confirmed by pathogens, 100 images diagnosed with typical viral pneumonia and 100 images of normal cases were collected. The design of the proposed model initially goes through a preprocessing of the input images followed by an expansion in information. Then, at that point, the model starts a stage to extricate the attributes followed by the learning step. At long last, the model starts a characterization and forecast process with a completely associated network framed of a few classifiers (Taigman et al., 2014). Profound learning and characterization were done utilizing the VGG convolutional neural organization (Richens, 2020).
The proposed model accomplished a precision of 92.5% in inward validation and 87.5% in outer validation. For the AUC basis, a worth of 97% in inside validation and 95% in outside validation were achieved. As to affectability model, we acquired a worth of 92% in inner approval and 87% in outer approval. The outcomes got by the model in the test stage show that the created model is extremely compelling in distinguishing COVID-19 and can be offered to health communities as a precise, rapid and effective clinical decision support system in COVID-19 detection (Hypponen et al., 2014).
Methodology
Publicly accessible dataset of blood smear images from both infected and non-infected malaria patients were obtained from Lister Hill National Center for Biomedical Communications (LHNCBC). Giemsa-stained thin blood smear slides from 150 Plasmodium Falciparum-infected and 50 non-infected patients were captured at Chittagong Medical College Hospital, Bangladesh. The dataset contained 27,558 cell images with the same number of malaria-infected and non-infected cell images which are 13,779 each (Rajaraman et al., 2018).
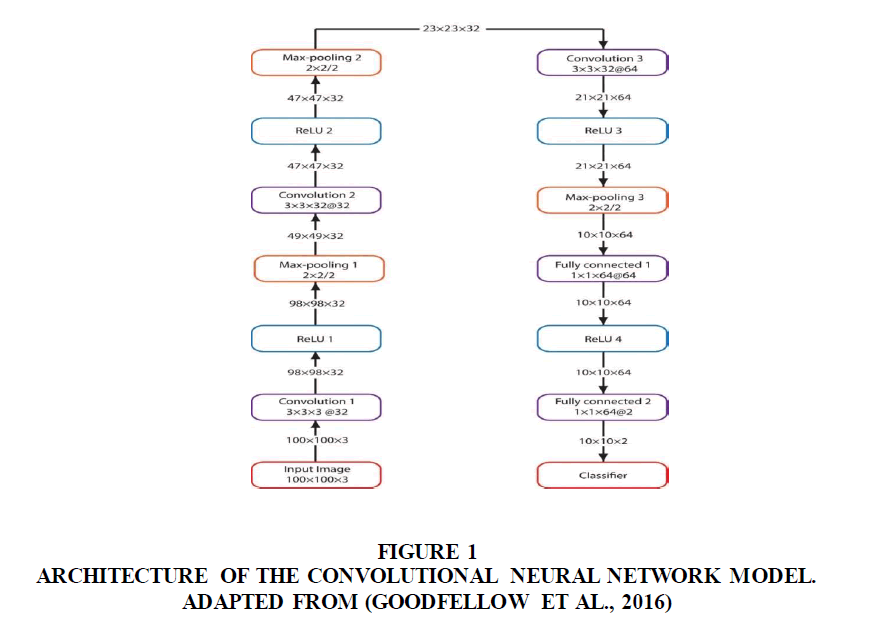
Convolutional Neural Networks (CNN) was used to train. The input to the CNN model formed sectioned cells of 100×100×3-pixel resolution having three convolution layers. Each convolution layer would use 3 × 3 filters with 2-pixel strides. The first and second convolutional layers would have 32 filters while the third convolutional layers would have 64 filters respectively. All convolutional layers would use ReLU activation. Stochastic Gradient Descent (SGD) (Corny et al., 2020) (Figure 1).
Figure 1: Architecture Of The Convolutional Neural Network Model. Adapted From (Goodfellow et al., 2016).
This shows various layers in the CNN, their input dimensions and output dimensions, the Rectified Linear Unit (ReLU) and the max-pooling layers used for the convolutional layers.
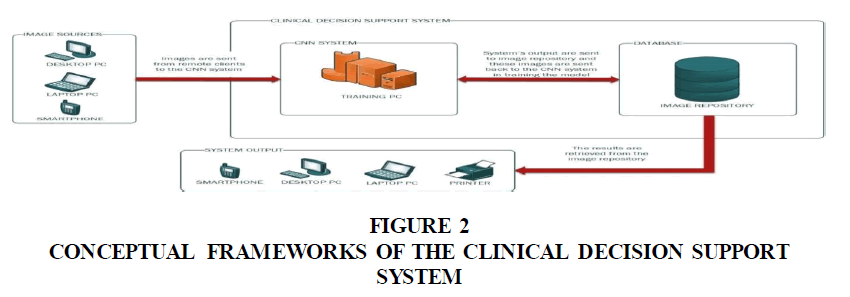
The Clinical Decision Support System (CDSS) consists of the main server that is comprised of a database for storing the classified images and a central PC for training the CNN model. The input and output of the system are connected to the main server through the internet (Figure 2). The patients digitalize blood smear images were used as input to the CDSS. The images were re-sampled to 100×100 pixels to match the dimensions of the dataset images that were used in training the CNN model. The output is a prediction as regard to the patient’s malaria status. These classified images are stored in the database which can be subsequently used to train the CNN-model. The results can be retrieved on various output devices connected to the system (Sandeep Kumar & Jayadev, 2020).
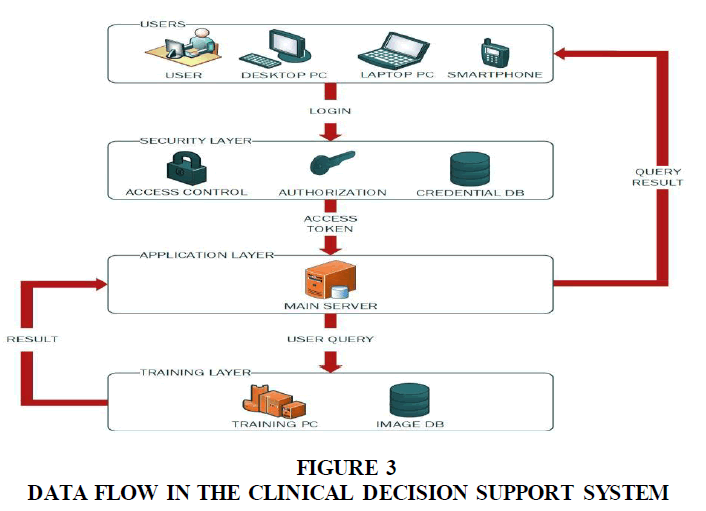
This shows the information one layer to another in the Clinical Decision Support System. The security layer controls access into the application system. The application layer sends user queries to the training layer and receives the results from the training layer. This result is subsequently sent to the privileged user by the application layer (Figure 3).
Results and Discussion
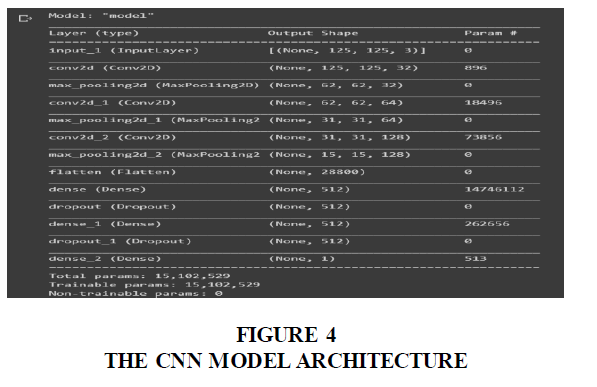
The layers of the CNN model were built using Keras which runs on Tensorflow. All layer specifications were done using Keras and the CNN model was compiled after.
The figure shows the CNN model architecture after all the layers were build using their respective specifications and compiled. The model was trained on the dataset using keras. Using specified batch size, epoch, class numbers and input dimensions. The model is the validated using the validate dataset and evaluate its performance (Figure 4).
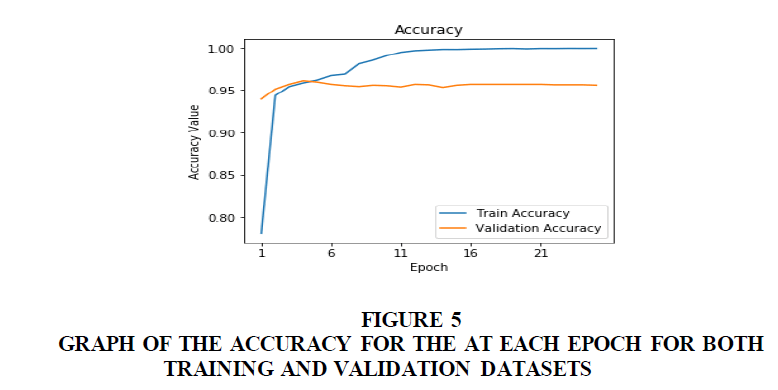
Figure 5 shows the plot of the accuracy of the model against the number of epoch. This increases as the model learns the dataset. The blue line is the graph for the training dataset and the orange line is for the validation dataset.
Figure 6 Graph of the accuracy for the at each epoch for both training and validation datasets. Figure 6 shows the plot of the accuracy of the model against the number of epoch. This increases as the model learns the dataset. The blue line is the graph for the training dataset and the orange line is for the validation dataset.
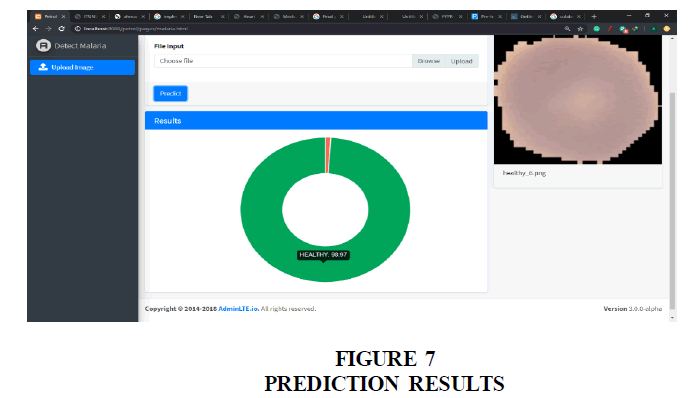
Figure 7 shows the result of the prediction displayed as a pie-chart with two variables. One for the infected probability and the other of the healthy probability. The variables are in red and green respectively.
Conclusion
With a majority of people infected by malaria in Nigeria being children below 5 years of age. Most children in this age group are generally unable to properly describe their symptoms to the medical professionals during diagnosis this has led to the conclusion to use a machine learning technique that can automate Polymerase Chain Reaction (PCR) and Rapid Diagnostic Tests (RDT) used to analyze blood smears images for malaria detection. Moreover, with a rapid and reliable diagnosis being a requirement for this system, training the model using convolutional neural networks is a proficient choice. The proficiency of convolutional neural networks in image classification delivering very accurate results makes it an adept selection for training the model. And with a very low doctor-to-patient ratio in Nigeria, this system would assist medical professionals receiving a potentially high amount of malaria-infected patients rapidly diagnose and assign priority to his/her patients.
This work will be deployed in a hospital where other e-health facilities will be fully integrated. Additional modules will be added to extent the existing medical personnel and patient’s information management system like the chatbot module that would help interact with patients.
References
Abiodun A.O., Olabode O.O, Sunmola Fatai & Emuoyibofarhe Ozichi Justice. (2021). Adaptive neuro-fuzzy expert systems for malaria parasite classification and severity level prediction. International Journal of Scientific & Engineering Research,12, Issue 2, ISSN 2229-5518.
Blobel, B., Goossen, W., & Brochhausen, M. (2014). Clinical modeling—a critical analysis.International journal of medical informatics,83(1), 57-69.
Indexed at, Google Scholar, Cross Ref
Bolaji, S.A. (2016). Addressing the Public Health Challenges Nigeria Faces.
Choi, G.H., Yun, J., Choi, J., Lee, D., Shim, J.H., Lee, H. C., & Kim, K.M. (2020). Development of machine learning-based clinical decision support system for hepatocellular carcinoma.Scientific reports,10(1), 1-10.
Indexed at, Google Scholar, Cross Ref
Corny, J., Rajkumar, A., Martin, O., Dode, X., Lajonchère, J.P., Billuart, O., & Buronfosse, A. (2020). A machine learning–based clinical decision support system to identify prescriptions with a high risk of medication error.Journal of the American Medical Informatics Association,27(11), 1688-1694.
Indexed at, Google Scholar, Cross Ref
Danyliv, A., Pavlova, M., Gryga, I., & Groot, W. (2013). Willingness to pay for physician services at a primary contact in Ukraine: results of a contingent valuation study.BMC Health Services Research,13(1), 1-11.
Indexed at, Google Scholar, Cross Ref
Dipanja, S. (2019). Detecting Malaria with deep learning.
Goodfellow, I., Bengio, Y., & Courville, A. (2016).Deep learning. MIT press.
Heyen, N.B., & Salloch, S. (2021). The ethics of machine learning-based clinical decision support: an analysis through the lens of professionalisation theory.BMC Medical Ethics,22(1), 1-9.
Indexed at, Google Scholar, Cross Ref
Hypponen, H., Reponen, J., Laaveri, T., & Kaipio, J. (2014). User experiences with different regional health information exchange systems in Finland.International Journal of Medical Informatics,83(1), 1-18.
Indexed at, Google Scholar, Cross Ref
Jiang, F., Jiang, Y., Zhi, H., Dong, Y., Li, H., Ma, S., & Wang, Y. (2017). Artificial intelligence in healthcare: past, present and future.Stroke and vascular neurology,2(4).
Indexed at, Google Scholar, Cross Ref
Karthikeyan, A., Garg, A., Vinod, P.K., & Priyakumar, U.D. (2021). Machine learning based clinical decision support system for early COVID-19 mortality prediction.Frontiers in public health,9, 626697.
Indexed at, Google Scholar, Cross Ref
Qjidaa, M., Ben-Fares, A., Mechbal, Y., Amakdouf, H., Maaroufi, M., Alami, B., & Qjidaa, H. (2020,). Development of a clinical decision support system for the early detection of COVID-19 using deep learning based on chest radiographic images. In2020 International Conference on Intelligent Systems and Computer Vision (ISCV), 1-6.
Indexed at, Google Scholar, Cross Ref
Rajaraman, S., Antani, S.K., Poostchi, M., Silamut, K., Hossain, M.A., Maude, R.J., & Thoma, G.R. (2018). Pre-trained convolutional neural networks as feature extractors toward improved malaria parasite detection in thin blood smear images.PeerJ,6, e4568.
Indexed at, Google Scholar, Cross Ref
Richens, J.G., Lee, C. M., & Johri, S. (2020). Improving the accuracy of medical diagnosis with causal machine learning.Nature communications,11(1), 1-9.
Indexed at, Google Scholar, Cross Ref
Sandeep Kumar, E., & Jayadev, S., P. (2020). Deep learning for clinical decision support systems: A review from the panorama of smart healthcare.Deep Learning Techniques for Biomedical and Health Informatics, 79-99.
Indexed at, Google Scholar, Cross Ref
Schroff, F., Kalenichenko, D., & Philbin, J. (2015). Facenet: A unified embedding for face recognition and clustering. InProceedings of the IEEE conference on computer vision and pattern recognition,815-823.
Indexed at, Google Scholar, Cross Ref
Taigman, Y., Yang, M., Ranzato, M.A., & Wolf, L. (2014). Deepface: Closing the gap to human-level performance in face verification. InProceedings of the IEEE conference on computer vision and pattern recognition, 1701-1708.
Indexed at, Google Scholar, Cross Ref
Teoh, E.R., & Kidd, D.G. (2017). Rage against the machine? Google's self-driving cars versus human drivers.Journal of safety research,63, 57-60.
Indexed at, Google Scholar, Cross Ref
Vasey, B., Ursprung, S., Beddoe, B., Taylor, E.H., Marlow, N., Bilbro, N., & McCulloch, P. (2021). Association of clinician diagnostic performance with machine learning–based decision support systems: a systematic review.JAMA network open,4(3), e211276-e211276.
Indexed at, Google Scholar, Cross Ref
Received: 04-Aug-2022, Manuscript No. JMIDS-22-12421; Editor assigned: 06-Aug-2022, PreQC No. JMIDS-22-12421(PQ); Reviewed: 20-Aug-2022, QC No. JMIDS-22-12421; Revised: 23-Aug-2022, Manuscript No. JMIDS-22-12421(R); Published: 30-Aug-2022