Research Article: 2024 Vol: 27 Issue: 1
Interrelationships amongst Water Quality Parameters and the Phytoplankton along a Salinity Gradient in the Lake Oloiden and the Lake Naivasha, Ramsar Site
Guto Kerubo Carolyne, Kisii University
Njiru Murithi James, Kisii University
Getabu Albert, Kisii University
Gichana Moraa Zipporah, Kisii University
Citation Information: Kerubo Carolyne, G., Murithi James, N., Albert, G., & Moraa Zipporah, G. (2024). Interrelationships amongst water quality parameters and the phytoplankton along a salinity gradient in the lake oloiden and the lake naivasha, ramsar site. Journal of Management Information and Decision Sciences, 27 (S1), 1-15.
Abstract
Lake Naivasha's water level increased and it merged with the Lake Oloiden; thus, forming an estuary; which may impact the water quality and phytoplankton community. The study was conducted with the aim of investigating the relationship amongst the water quality parameters and the phytoplankton along a salinity gradient in the Lakes Naivasha and Oloiden. All water quality parameters were measured insitu and phytoplankton were sampled. Lake Oloiden's water quality were significantly higher than Lake Naivasha's except for the dissolved oxygen. The pH positively correlated with all water quality parameters in the Lakes Oloiden and Naivasha except the later's dissolved oxygen. Euglenophycea negatively correlated with Cyanophycea and Bacillariophycea in the Lake Naivasha while it positively correlated with Chlorophycea and Cyanophycea and negatively correlated with Dinophycea and Bacillariophycea in the Lake Oloiden. Chlorophycea had a positive correlation with all water quality parameters in the Lakes Oloiden and Naivasha except for the dissolved oxygen in both; temperature and pH for the later. All water quality parameters had a positive association with the salinity and total dissolved solids. Rhodophycea was positively affected by the salinity and conductivity. Chlorophycea was positively affected by total dissolved solids, conductivity and salinity while Dinophycea was negatively affected by the temperature and pH in the Lakes Naivasha and Oloiden. Lake Oloiden's Cyanophycea was negatively affected with the dissolved oxygen while it's effect was positive in the Lake Naivasha.
Keywords
Bacillariophycea, Chlorophycea, Cyanophycea. Estuarine, Euglenophycea, Pearson correlation.
Introduction
Lake Naivasha has undergone drastic ecological changes due to water levels fluctuations (Omondi et al., 2020). The water level increased, leading to its merging with Lake Oloiden (saline) (Ballot et al., 2009; Nyangau, 2021). This occurrence resulted in the formation of an estuary; which has unique physical, chemical and biological features: distinguished by a salinity gradient. It's biotic and abiotic aspects show non-linear variation. It may favor a high rate of productivity; due to continuous flow of allochthonous organic matter (Telesh & Khelebivich, 2010). Phytoplankton are major primary producers in an aquatic environment. Their distribution (spatial and temporal) and abundance is affected by abiotic factors; thus, hydrographical factors cause a differential effect (Kathiresan, 2013). Abiotic factors were associated with the phytoplankton community in an experiment. Salinity and other abiotic stressors were manipulated (changes in salinity and other factors e.g., temperature and pH) in the inland and other transitional environment to produce an effect on growth performance and frequency of their joint effect. The stressors had an effect on individual species and the community composition. A higher salinity led to a decrease in the density of Daphnia magna; although, the temperature had no effect. The decreased in abundance affected the phytoplankton community structure. Thus, Salinity, pH and temperature are key abiotic stressors in an aquatic ecosystem (Velasco et al., 2019).
Some Lake Naivasha sites may be salinized due to its merging with the Lake Oloiden. Fresh water organisms may have varying sensitivity towards salinity stress which may alter species and community composition. The water quality deteriorated due to over-abstration of water from the Lake Naivasha and the salinization of the Lake Oloiden; leading to changes also in the phytoplankton community. Lake Naivasha’s phytoplankton were previously dominated by Cyanophycea but shifted to Chlorophycea while the Lake Oloiden were previously dominated by the Chlorophycea but shifted to the Cyanophycea. Changes in the physical and chemical properties in an aquatic ecosystem are interlinked with the composition of phytoplankton. Due to the merging of the Lake Naivasha and the Lake Oloiden there may be changes in the abiotic factors and the phytoplankton community (Ballot et al., 2009; Rochelle-Newall et al., 2011). The objective of the study was to investigate the relationship amongst water quality parameters and the phytoplankton along a salinity gradient in the Lakes Naivasha and Oloiden.
Material and Methods
Study Area
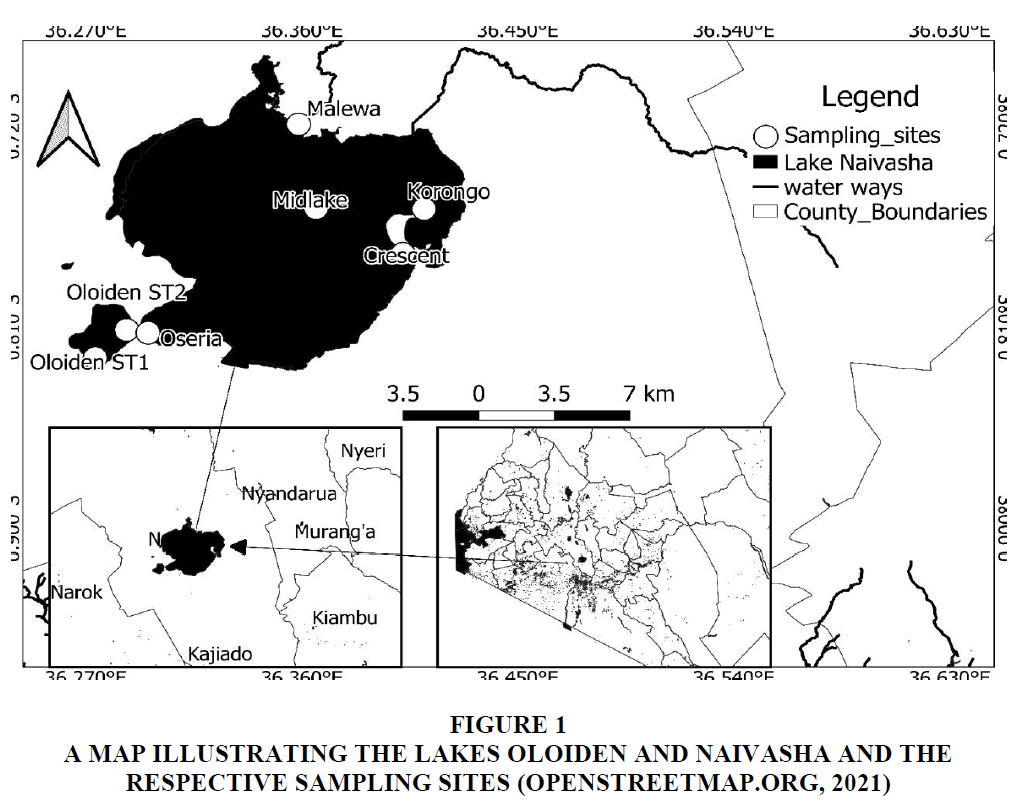
The study was done for one year (August, 2020 to July, 2021), along a salinity gradient in the Lake Oloiden (00o50’S, 36o17’E) and the Lake Naivasha (00o46’S, 36o22’E) (Ballot et al., 2009). A transect was drawn from Oloiden ST1 to Malewa and two sites (Crescent and Korongo) were added in the sampling to cover the Lake Naivasha. Oloiden STI was the first site while Oloiden ST2 was the second and it bordered Oseria. The Oloiden ST2 and Oseria are estuarine since they merged due to the high water level. Midlake was the 4th site; an open water site while Malewa was an edge site where River Malewa drains into the Lake Naivasha. The 6th site was a crater lake that also merged with the Lake Naivasha due to high water levels. Korongo was the 7th site; a lagoon that is connected to the Lake Naivasha (Nyangau, 2021).
Water Quality
The temperature, dissolved oxygen, conductivity, total dissolved solids, salinity and pH were measured insitu in all sites using a YSI Multiparameter meter figure 1 (YSI Professional Plus) (Nyangau, 2021).
Figure 1 A map illustrating the Lakes Oloiden and Naivasha and the Respective Sampling Sites (Openstreetmap.Org, 2021)
Phytoplankton
Phytoplankton sample was picked by a van don sampler in duplicate: sieved through a phytoplankton net (30 μm) and preserved by drops of Lugol iodine solution. Identification and counting were done in Sedgewick Rafter cell, under a compound microscope (×100) and keys were utilized (Suthers et al., 2019; Bellinger & Sigee, 2015). The density (individuals per litre) of the phytoplankton was calculated: where, the average number of individuals counted in the sample was divided by the volume of water in which the sample was obtained.
Results
There were significant differences in temperature with respect to sites (P<0.05) and the Midlake's was the highest Table 1. All study site's dissolved oxygen were significantly different (P<0.05): Korongo and Midlake's were outstanding. The conductivity, salinity, pH and total dissolved solids were significantly different among the study sites (P<0.05); Oloiden ST1 and ST2 were higher.
| Table 1 Water Quality Parameters in the Lake Naivasha and Lake Oloiden Study Sites | |||||||
| Oloiden ST1 | Oloiden ST2 | Oseria | Crescent | Korongo | Midlake | Malewa | |
| Temperature (°C) | 21.96 ± 1.12 | 21.12 ± 3.25 | 20.8 ± 2.8 | 21.39± 0.67 | 21.74 ± 1 | 20.08± 3.84 | 21.97 ± 1.23 |
| Dissolved oxygen (mg/l) | 6.29 ± 2.2 | 6.57 ± 1.3 | 5.95 ± 1 | 5.87 ± 0.9 | 5.88± 0.7 | 6.73 ± 1.3 | 6.54N± 0.8 |
| Conductivity (µS/m) | 0.58 ± 0.1 | 0.58 ± 0.1 | 0.19 ± 0.04 | 0.17 ± 0.4 | 0.19± 0.4 | 0.18 ± 0.04 | 0.18 ± 0.04 |
| Salinity (ppt) | 0.31± 0.07 | 0.31 ± 0.06 | 0.096± 0.02 | 0.096± 0.02 | 0.09± 0.02 | 0.096± 0.02 | 0.093± 0.02 |
| pH | 8.56 ± 0.2 | 8.46 ± 0.4 | 7.68 ± 0.04 | 7.45 ± 0.3 | 7.62± 0.2 | 7.65 ± 0.2 | 7.52± 0.2 |
| TDS (mg/l) | 0.41± 0.09 | 0.41 ± 0.08 | 0.13 ± 0.02 | 0.12 ± 0.02 | 0.13± 0.02 | 0.12 ± 0.02 | 0.15± 0.1 |
The Lake Oloiden had a significantly higher: temperature, conductivity, salinity, pH and total dissolved solids (P<0.05) Table 2.
| Table 2 Water Quality Parameters in the Lake Naivasha and Lake Oloiden (Cond=Conductivity) | ||||||
| Temperature (o C) | Dissolved oxygen (mg/l) | Cond (µS/m) | Salinity (ppt) | pH | TDS (mg/l) | |
| L. Oloiden | 21.54 ± 2.19 | 9.58 ± 1.75 | 0.58 ± 0.1 | 0.31 ± 0.065 | 8.51 ± 0.3 | 0.41± 0.085 |
| L. Naivasha | 21.12 ± 1.91 | 6.19 ± 0.94 | 0.18 ± 0.14 | 0.096 ± 0.02 | 7.58± 0.26 | 0.037± 0.09 |
The species were significantly different with respect to the study sites. Oseria, Crescent, Korongo, Midlake, Malewa, Oloiden ST1 and Oloiden ST2 had varied number of species; 26, 25, 24, 23, 22, 14 and 13 respectively Table 3. Euglena mutabilis, Ceratium hirundinella, Scenedesmus Opoliensis and Surirella linearis had similarities. Chlorophycea had the highest number of species; it was followed by Bacillariophycea and the Cyanophycea.
| Table 3 Phytoplankton Species in the Respective Sites in Lake Naivasha and Lake Oloiden | |||||||
| Phytoplankton species | Oseria | Crescent | Korongo | Midlake | Malewa | Oloiden ST1 | Oloiden ST2 |
| Bacillariophycea | |||||||
| Aulacoseira granulata | + | + | + | + | + | + | + |
| Nitzschia sp. | + | + | + | + | + | + | + |
| Navicula subtilissima | + | - | + | + | + | - | - |
| Tetracyclus sp. | + | + | + | + | + | + | + |
| Chlorophycea | |||||||
| Surirella elegans | + | + | + | + | + | + | + |
| Surirella linearis | + | + | - | + | - | + | - |
| Surirella minuta | + | - | + | + | - | - | - |
| Chlorella sp. | + | + | + | + | + | + | - |
| Crucigenia tetrapedia | + | - | + | - | - | - | - |
| Scenedesmus communis | + | + | + | + | + | + | + |
| Scenedesmus opoliensis | + | + | + | + | + | + | - |
| Scenedesmus accuminatus | + | + | + | + | + | + | + |
| Spirogyra prolecta | - | + | - | - | - | + | - |
| Pediastrum v. boryanum | + | - | + | + | + | + | + |
| Pediastrum boryanum | + | + | + | + | + | - | + |
| Pediastrum duplex | - | - | + | + | + | + | + |
| Pediastrum simplex | - | - | + | - | - | + | - |
| Tetraedron sp. | - | - | + | - | - | - | - |
| Tetmemorus sp. | + | + | - | - | - | - | - |
| Ankistrodesmus falcatus | + | + | + | - | - | - | - |
| Oocystis sp. | - | - | + | - | - | - | - |
| Coelastrum sp. | - | - | - | - | - | + | - |
| Westella sp. | - | + | - | - | - | + | - |
| Actinastrum sp. | + | - | - | - | - | - | - |
| Cyanophycea | |||||||
| Anabaena circunalis | + | + | + | + | + | + | + |
| Athrospira sp. | + | + | - | + | - | + | + |
| Cylindrospermum sp. | + | + | + | + | + | + | + |
| Chroococcus turgidus | + | + | - | + | - | + | + |
| Microsistis sp. | + | + | + | + | + | + | + |
| Dinophyceae | |||||||
| Ceratium hirundinella | + | - | + | + | + | + | + |
| Ceratium cornutum | - | - | - | + | + | + | + |
| Lepocinclis sp. | + | + | + | + | + | + | + |
| Euglenophycea | |||||||
| Euglena mutabilis | - | - | + | + | + | + | + |
| Phacus helicoides | - | + | + | - | + | - | - |
| Peridinium cinctum | + | + | + | + | + | + | + |
| Rhodophycea | |||||||
| Hildenbrandia sp. | + | + | + | + | + | - | - |
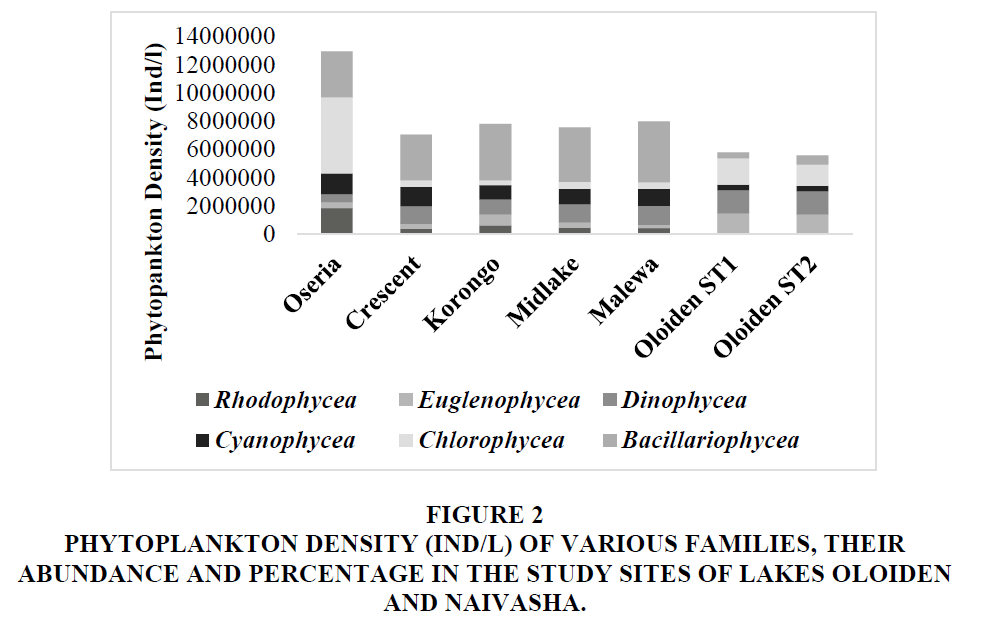
Oseria had the highest cumulative density for all the phytoplankton families. Lake Naivasha sites had the highest density in Bacillariophycea followed by Chlorophycea Figure 2. Chlorophycea had the highest density; it was followed by Dinophycea in the Lake Oloiden. Overall, Bacillariophycea had the highest abundance and percentage while Rhodophycea had the lowest.
Figure 2 Phytoplankton Density (IND/L) of Various Families, their Abundance and Percentage in the Study Sites of Lakes Oloiden and Naivasha.
The temperature correlated positively with the dissolved oxygen, conductivity, total dissolved solids and pH in the Lake Oloiden (P=0.05*, P=0.01**). Dissolved oxygen's correlation was positive with the temperature and negatively correlating with the salinity, total dissolved solids and pH. The conductivity correlated positively with the temperature, salinity and TDS while it correlated negatively with the dissolved oxygen.
The total dissolved solids correlated positively with the conductivity, salinity, temperature and pH. Salinity correlated negatively with the dissolved oxygen and positively correlated with the conductivity, TDS and pH. The pH's correlation was positive with the temperature, conductivity, TDS and salinity.
Euglenophycea correlated negatively with Dinophycea and Bacillariophycea while it positively correlated with Chlorophycea and Cyanophycea. Dinophycea's correlation was negative with Euglenophycea, Chlorophycea and Cyanophycea while it correlated positively with Bacillariophycea.
Cyanophycea correlated positively with Euglenophycea and Chlorophycea; it negatively correlated with Dinophycea and Bacillariophycea. Chlorophycea correlated positively with Euglenophycea and Cyanophycea while it negatively correlated with Dinophycea and Bacillariophycea. Bacillariophycea correlated positively with Dinophycea and negatively correlated with Euglenophycea, Chlorophycea and Cyanophycea.
The conductivity, total dissolved solids, salinity and pH had a significant positive correlation with Euglenophycea density while dissolved oxygen’s was negative. Temperature, conductivity, total dissolved solids, salinity and pH’s correlation with the density of Dinophycea was negative while dissolved oxygen’s was positive.
Dissolved oxygen negatively correlated with the density of Cyanophycea; it's correlation was positive with the conductivity, total dissolved solids, salinity and pH. All the variables had a positive correlation with Chlorophycea density with exception of the dissolved oxygen. The dissolved oxygen and conductivity had a positive correlation with the Bacillariophycea density and negative with the temperature, total dissolved solids, salinity and pH Table 4.
| Table 4 Pearson’s Correlation of Phytoplankton Families with water Quality Parameters in the Lake Oloiden (Temp=Temperature, DO=Dissolved Oxygen). | |||||||||||
| Eglenophycea | Dinophycea | Cyanophycea | Chlorophycea | Bacillariophycea | Temp | DO | Cond | TDS | Salinity | pH | |
| Euglenophycea | 1 | -1** | 1** | 1** | -1** | 1** | 1** | 1** | 1** | 1** | 1** |
| Dinophycea | -1** | 1 | -1** | -1** | 1** | -1** | 1** | -1** | -1** | -1** | -1** |
| Cyanophycea | 1** | -1** | 1 | 1** | -1** | 1** | 1** | 1** | 1** | 1** | 1** |
| Chlorophycea | 1** | -1** | 1** | 1 | -1** | 1** | -1** | -1** | -1** | -1** | -1** |
| Bacillariophycea | -1** | 1** | -1** | -1** | 1 | -1** | 1** | -1**- | -1** | -1** | -1** |
| Temp (o C) | 1 | -1** | 1 | 1** | -1** | 1 | 0.26** | 0.14** | 0.1** | 0.31 | 0.29** |
| DO (mg/l) | -1** | 1** | -1** | -1** | 1** | 0.26** | 1 | -033** | -0.35 | -0.38** | -0.15 |
| Cond (µS/m) | 1** | -1** | 1** | 1** | 1** | 0.14** | -0.3 | 1 | 0.93** | 0.95** | 0.166** |
| TDS (mg/l) | 1** | -1** | 1** | 1** | -1** | 0.1** | -0.35** | 0.93** | 1 | 0.93** | 0.135** |
| Salinity (ppt) | 1** | -1** | 1** | 1** | -1** | 0.31 | -0.38** | 0.95** | 0.93** | 1 | 0.152** |
| pH | 1** | -1** | 1** | 1** | -1** | 0.29** | 0.15** | 0.17 | 0.14** | 0.152** | 1 |
Lake Naivasha’s temperature correlated positively with the dissolved oxygen, conductivity, TDS, salinity and pH (P=0.05*, P=0.01**). The dissolved oxygen correlated positively with temperature and pH while it negatively correlated with conductivity, TDS and salinity. Conductivity's correlation was positive with the TDS, salinity, pH and temperature. Total dissolved solids correlated negatively with the dissolved oxygen while it positively correlated with the pH, salinity and conductivity.
The salinity correlated positively with temperature, conductivity, TDS and DO similar to the pH's correlation with the temperature, DO, TDS, conductivity and salinity Table 5.
| Table 5 Pearson’s Correlation of Phytoplankton Families with Water Quality Parameters in the Lake Naivasha | ||||||||||||
| Rhodophycea | Euglenophycea | Dinophycea | Cyanophycea | Chlorophycea | Bacillariophycea | Temp | DO | Cond | TDS | Salinity | pH | |
| Rhodophycea | 1 | 0.052 | -0.97** | 0.6 | 0.986** | -0.53 | 0.6 | 0.56 | 0.88 | 0.78 | 0.86* | 0.75 |
| Euglenophycea | 0.052 | 1 | 0.63 | -0.93** | 0.034 | 0.913** | -0 | -0.51 | -0.26 | -0.2 | -0.09 | -0.58 |
| Dinophycea | 0.97** | 0.63 | 1 | -1 | -0.59 | -0.6 | -0.79* | -0.79* | -0.64 | -0.6 | -0.54 | -0.81* |
| Cyanophycea | 0.65 | -0.93** | -0.83* | 1 | 0.2 | 0.83* | -0.75* | 0.78* | 0.43 | 0.35 | 0.23 | 0.62 |
| Chlorophycea | 0.99* | 0.034 | -0.6 | 0.2 | 1 | -0.21 | -1 | 0.43 | 0.8* | 0.72* | 0.83** | 0.48 |
| Bacillariophycea | -0.53 | -0.91** | -0.6 | 0.83* | -0.21 | 1 | 0.3 | 0.37 | 0.53 | -0.1 | -0.07 | 0.64 |
| Temp (o C) | 0.598 | -0.494 | -0.749* | 0.747* | 0.535 | 0.27 | 1 | 0.23** | 0.22** | 0.21 | 0.71** | 0.3** |
| DO (mg/l) | 0.555 | -0.511 | -0.8 | 0.777* | 0.43 | 0.37 | 0.23* | 1 | -0.08 | -0.08** | 0.09** | 0.19** |
| Cond (µS/m) | 0.882* | -0.255 | -0.6 | 0.4 | 0.796 * | 0.53 | 0.22** | -0.078** | 1 | 0.82** | 0.82** | 0.85** |
| TDS (mg/l) | 0.776 | -0.237 | 0.57 | 0.3 | 0.72* | 0.09 | 0.21** | -0.082** | 0.99** | 1 | 0.99** | 0.7** |
| Salinity (ppt) | 0.862* | 0.092 | -0.5 | 0.2 | 0.833** | 0.07 | 0.17** | -0.092** | 0.99** | 0.99** | 1 | 0.68** |
| pH | 0.748 | -0.576 | -0.805* | 0.6 | 0.478 | 0.64 | 0.3** | 0.19** | 0.7** | 0.7** | 0.68** | 1 |
Rhodophycea correlated positively with Dinophycea and Chlorophycea while Euglenophycea's correlation was negatively with Cyanophycea and Bacillariophycea. Dinophycea's correlated negatively with Rhodophycea and Cyanophycea. Cyanophycea correlated negatively with Euglenophycea while it positively correlated with Bacillariophycea.
The correlation of Chlorophycea with Rhodophycea was positive; similarly, to Bacillariophycea and Euglenophycea.
Salinity and conductivity had a significant positive correlation with Rhodophycea density. The temperature and pH negatively correlated with the density of Dinophycea. Cyanophycea density positively correlated with the temperature and dissolved oxygen. The conductivity, TDS and salinity positively correlated with the density of Chlorophycea.
Discussion
All the study sites had a temperature >20°C and Oloiden ST1 and ST2's were higher; which could be attributed to their higher salinity. There were variation in the dissolved oxygen among study sites; although, it was lower than the previous findings and similar in both lakes. The total dissolved solids, salinity, temperature, conductivity and pH were higher in the Lake Oloiden sites as compared to the Lake Naivashas'. Conductivity was much lower than in the previous findings similar to the Lake Oloiden's pH; which could be attributed to dilution due to the increased water level (Ballot et al., 2009; Ndungu et al., 2014).
There were 32 phytoplankton species that were identified in the Lakes Naivasha and Oloiden with an average of 24 and 13 species respectively; which was lower than previous research findings (Omondi et al., 2020; Ballot et al., 2009).
The number of species decreased with the increase in the salinity (Larson & Belovsky, 2013). Oseria had the highest number of phytoplankton species while Oloiden ST1 and ST2 had the lowest. The Lake Naivasha had the highest abundance in Bacillariophycea, followed by Dinophycea and then Cyanophycea (Hubble & Harper, 2002) while the Lake Oloiden's were Chlorophycea and followed by Dinophycea; findings that contrasted with the previous (Ballot et al., 2009). Presence of diatoms (Bacillariophycea) and their highest density (in Lake Naivasha) was indicative of moderate to high nutrient condition. Dinophycea presence and its 2nd in abundance in both lakes was an indicator of accumulated organic matter (Hubble & Harper, 2002).
Spatial variation of phytoplankton is of importance for water quality monitoring; since they are governed by various physico-chemical parameters. The changes in an ecosystem may be noted by the species composition and their density (Kathiresan, 2013). Aulacoseira granulata was present only in Lake Naivasha and it's density was high: which was indicative of a eutrophic condition; which may have stimulated the growth of Microcystis sp. (blooms were noted and it's abundance was highest in the Cresent) (Hubble & Harper, 2002; Ballot et al. 2009). Tetracyclus sp. was present in both lakes; although Oloiden's density were the lowest. This may have been influenced by the salinity and other water quality variables (Telesh & Khelebivich, 2010). Changes may occur to individual organisms primarily and may extend to the community composition (Velasco et al., 2019).
An increase in the temperature led to an increase in the dissolved oxygen, conductivity, total dissolved solids and pH in the Lake Oloiden. Increase in temperature allows phytoplankton to photosynthesize; producing oxygen and hydroxide ions.
The increase in hydroxide ions may have led to an increase in the pH and total dissolved solids which in turn led to an increase in the conductivity (Omondi et al., 2020). On the other hand, increase in the dissolved oxygen led to an increase in temperature and a decrease in salinity and total dissolved solids and pH. At high temperatures, photosynthesis may take place leading to an increase in the dissolved oxygen. However, high temperatures may affect the solubility of carbon dioxide; leading to a decrease in the pH and TDS (Omondi et al., 2020). The effect on salinity could be attributed to changes that take place in an estuarine ecosystem (Oloiden ST2) where: cation and anion relative proportion changes non-linearly; since salinity is supposed to increase as temperature increases (Nielsen et al., 2003).
The increase in the conductivity led to an increase in the temperature, salinity and TDS while the dissolved oxygen decreased. Decreased dissolved oxygen could be due to decomposition/respiration; which in turn produces hydrogen ions that could lead to an increment in TDS and conductivity (Ndungu et al., 2014). Conductivity could be directly related to conductive ions from inorganic material and dissolved salt contributing to salinity of the water. Total dissolved solids increased as conductivity, pH, salinity and temperature increased. An increment of ions could allow more electrical conductivity (increasing conductivity) increasing the temperature, pH and salinity (Nielsen et al., 2003). High total dissolved solids; especially, for Oloiden ST2 could be due to an increase in ionic composition due to; complexity in the biogeochemical processes that occur in an estuary (Telesh & Khelebivich, 2010). Salinity increase led to a decrease in the dissolved oxygen and an increase in the conductivity, TDS and pH. The increasing in carbon dioxide during respiration may have led to the decrease in the pH (Omondi et al., 2020). A pH increase led to an increase in the temperature, conductivity, TDS and salinity. This was witnessed in the Lake Oloiden; where these variables were high and salinity could be the influencing factor (Nielsen et al., 2003).
Lake Naivasha’s temperature increase led to an increase in the dissolved oxygen, conductivity, TDS, salinity and pH. This was because of photosynthesis that may take place; thus, leading to the production of oxygen and hydroxide ions consequently, leading to the increase in the variables (Kathiresan, 2013; Omondi et al., 2020). An increase in the conductivity led to an increase in pH, TDS, salinity and temperature. Increase in dissolved oxygen led an increase in temperature and pH and a decrease in the conductivity, TDS and salinity. Total dissolved solids increase led to a decrease in the dissolved oxygen and an increase in the pH, salinity and conductivity in line with previous findings. TDS is composed of ions and they may contribute in increased conductivity, pH and the salinity (Nielsen et al., 2003). pH increment led to an increase in the temperature, DO, TDS and salinity. The pH increment may have been to increase in hydroxide ions which may have led to increase in TDS and salinity; thus, allowing an increase in the temperature (Omondi et al., 2020).
Oseria had the highest number of species while Oloiden ST2 had the lowest yet, both were estuarine. Although Oloiden ST2’s salinity was higher. The highest cumulative density in all the families was in Oseria. An estuarine environment could be characterized with a higher phytoplankton productivity (Larson and Belovsky, 2013). All the species belonging to Bacillariophycea were present in all the sites with the exception of Navicula subtilissima that was absent in the Crescent and Oloiden ST1. Thus, Bacillariophycea could have had a wide tolerance to variation (Olofson et al., 2020; Nielsen et al., 2003). Although, it's species were different from the previous findings with the exception of Aulacoseira granulata (Hubble and Harper, 2002; Ballot et al., 2009) and Navicula (Hubble and Harper, 2002).There was variation in the species belonging to Chlorophycea: Chlorella sp. was present in all the sites except in the Oloiden ST2; similar to Pediastrum v. boryanum except in the Crescent. Pediastrum duplex was also present in all the sites except in Oseria (estuarine) and Crescent (only site that had Tetmorus sp). Tetraedron and Oocytis sp. were only present in Korongo while Actinastrum sp. was present only in Oseria and Coelastrum sp. was only present in the Oloiden ST1. Chlorella, Scenedesmus sp. (Hubble and Harper, 2002) and Pediastrum simplex (Ballot et al., 2009) were similar to previous Chlorophycea findings. Prevailing abiotic conditions may have an effect on the phytoplankton; Crescent is a crater lake while Oseria and Oloiden ST2 were estuarine (Telesh & Khelebivich, 2010).
All the species belonging to Cyanophycea were present in all the sites with exception of Chroococcus turgidus and Athrospira sp. that were absent in Korongo and Malewa. All the species belonging to Cyanophycea were dissimilar to the previous findings except Microcystis and Athrospira sp. (Ballot et al., 2009).
Lepocinclis sp. was the only species of Dinophycea that was present in all the study sites and only Ceratium was similar to the previous findings (Hubble & Harper, 2002). Euglenophycea; Peridinium cinctum was present in all the sites. Rhodophycea had one species that was present in all the Lake Naivasha sites namely: Hildenbrandia. Variation in the species has been observed previously in Lake Naivasha and Oloiden. This was due to Limnological, hydrobiological and ecological changes (Omondi et al., 2020). There may be change in species and diversity pattern with the change in abiotic conditions (Larson & Belovsky, 2013). Change in Limnological attributes may be influenced by rainfall fluctuations and catchment activities i.e., agricultural (Hubble & Harper, 2002).
The Lake Oloiden had Chlorophycea was the most abundant that was followed by Dinophycea contrary to previous findings (Ballot et al., 2009). Oloiden ST1 had the dominance of Chlorophycea while Oloiden ST2’s was Dinophycea. Overall, Dinophycea was second in dominance tallying with (Nielsen et al., 2003). Its presence was signifying an accumulation of organic matter; which was a sign of a eutrophic condition (Hubble & Harper, 2002; Rayori et al., 2021). Organic matter accumulation could be due to the decomposition of acacia trees (Acacia xanthophloe) that fell into the water post water level rise and also from the river discharge. The Lake Naivasha had the dominance of Bacillariophycea that was followed by Chlorophycea and Cyanophycea contrary to the finding previously (Ballotet al., 2009; Omondi et al., 2020). Crescent, Korongo, Midlake, Malewa had Bacillariophycea with the high density as compared to Lake Oloiden sites that were low, contrary to (Olofsson et al., 2020).The highest density was in Malewa which could be attributed to high nutrients that may be entering the lake via River Malewa from the agricultural catchment area (Ndungu et al., 2014).
An increase in Euglenophycea led to a decrease in Dinophycea and Bacillariophycea but an increase in Chlorophycea and Cyanophycea in the Lake Oloiden. This was observed in the abundance of Bacillariophycea which was in line with Olofsson et al. (2020) similar to Chlorophycea and contrary for Dinophycea and Cyanophycea. The density of Cyanophycea was low: could be due to less adaptability to salinity while Chlorophycea and Dinophycea’s density were high; due to the prevailing conditions that may have favored them (Telesh & Khelebivich, 2010; Olofsson et al., 2020).
The increase of in Dinophycea led to the decrease in Euglenophycea, Cyanophycea and Chlorophycea while Bacillariophycea increased. On contrary, Euglenophycea density was highest in the Lake Oloiden while Oloiden ST2's was higher than Oseria’s; thus, exhibiting non- linear variation in an estuary (Rochelle-Newall, 2011). As Cyanophycea increased Dinophycea and Bacillariophycea decreased but Euglenophycea and Chlorophycea increased. The density finding tallied with Chlorophycea and Euglenophycea while it was contrary for Dinophycea, since it was high (Olofson et al., 2020). An increase in Chlorophycea led to an increase in Euglenophycea and Cyanophycea but a reduction in Dinophycea and Bacillariophycea. Density results showed contrast in Cyanophycea (Telesh & Khelebivich, 2010; Olofson et al., 2020). Increase in Bacillariophycealed to an increase in Dinophycea and a decrease in Euglenophycea and Chlorophycea and Cyanophycea. On the contrary, Bacillariophycea density was lowest in Oloiden ST1 and ST2. Changes in abiotic factors may influence phytoplankton species and finally to the family level (Flöder et al., 2010).
In the Lake Naivasha: an increase in Rhodophycea led to an increase in Dinophycea and Chlorophycea. The density of Dinophycea tallied while there was a contrast in the density of Chlorophycea except for Oseria (had the highest while other sites were low). Dinophycea density was high in all the sites except in Oseria (showed non-linear variation in an estuary) (Nielsen et al., 2003; Olofson et al., 2020). Euglenophycea increase led to a decrease in Cyanophycea and Bacillariophycea. The density of Bacillariophycea and Cyanophycea were high: thus, density results showed contrast. Dinophycea increased as Rhodophycea and Cyanophycea decreased. The density resulted tallied for Rhodophycea and Cyanophycea except for the earlier's in Oseria (was the highest). Cyanophycea increased with the decrease in Euglenophycea and Bacillariophycea. Density results showed a tally with Euglenophycea and a contrast with Bacillariophycea. An increase in Chlorophycea led to an increase in Rhodophycea while increase in Bacillariophycea led to an increase in Euglenophycea. Results contrasted for all the families except Oseria's Rhodophycea. Rhodophycea and Chlorophycea density were high in Oseria as compared to the other 4 sites; an estuarine environment may have high productivity (Olofson et al., 2020). The family that takes dominance may be well adapted to the prevailing conditions since they may exhibit compensatory growth.
The conductivity, total dissolved solids, salinity and pH had a significant positive effect on Euglenophycea density while dissolved oxygen's was negative in the Lake Oloiden. Its abundance was highest in Lake Oloiden; variables that had a positive effect were high. Oloiden ST2’s density was higher as compared to Oseria and yet both were estuarine. Thus, the non-linearlity in productivity was noted in an estuary (Nielsen, D. L., Brock, M. A., Rees, G. N., & Baldwin, D. S. (2003).; Telesh & Khelebivich, 2010). Temperature, conductivity, total dissolved solids, salinity and pH’s effect on the density of Dinophycea was negative while dissolved oxygen's was positive. Variables that had a negative effect were high in Lake Oloiden; although, on the contrast it's density was high (Olofsson et al., 2020). The density of Dinophycea and Euglenophycea increased with the dissolved oxygen; thus, when the phytoplankton density is high the DO is bound to increase due to photosynthesis (Omondi et al., 2020).
All the variables had a positive effect on Chlorophycea density with exception of the dissolved oxygen in Lake Oloiden. It's density was high in Lake Oloiden sites; the abiotic could have been favorable (Ballot et al., 2009; Omondi et al., 2019).
The dissolved oxygen and conductivity had a positive effect on the Bacillariophycea density while the temperature, total dissolved solids, salinity and pH had a negatively affect. Its density was low in Lake Oloiden sites: thus, water quality parameters may have a negative effect on phytoplankton (Nielsen et al., 2003). Salinity and temperature are major environmental variables that situate phytoplankton into context (Suther et al., 2019). Phytoplankton may experience varying sensitivity towards salinity stress; may alter community composition.
Rhodophycea was positively influenced by the Conductivity and salinity in the Lake Naivasha. Most site's density was low except for Oseria; that was estuarine and thus, supported higher productivity (Telesh & Khelebivich, 2010; Olofsson et al., 2020). The temperature and pH had a negative effect on the density of Dinophycea in Lake Naivasha. Thus water quality may negatively affect phytoplankton; although, it's density was high in most sites with the exception of Oseria (lowest) (Omondi et al., 2020). The density of Chlorophycea was positively affected by: the conductivity, total dissolved solids and salinity. It's density was low in most sites except for Oseria (Ballot et al., 2009). Cyanophycea density was affected positively by temperature and dissolved oxygen. It's density was high. Temperature influences photosynthetic activity of phytoplankton: directly proportional (temperature increase led to an increase in the productivity). Each individual phytoplankton is temperature adapted (Velasco et al., 2019).
Conclusion
All water quality parameters had a positive association with the salinity and total dissolved solids. Rhodophycea was positively affected by the salinity and conductivity. Chlorophycea was positively affected by total dissolved solids, conductivity and salinity while Dinophycea was negatively affected the temperature and pH in the Lakes Naivasha and Oloiden. Lake Oloiden's Cyanophycea was negatively affected with dissolved oxygen while it's effect was positive in the Lake Naivasha. There was variation in the interrelationships amongst water quality and phytoplankton along the salinity gradient.
Acknowledgement
The study was undertaken in Lake Naivasha through the Kenya Marine and Fisheries Research Institute (KMFRI), Naivasha station. Our gratitude is extended to all the staff for aiding in acquirement of samples space for laboratory analysis.
Conflict of Interest
All the authors have no conflict of interest on the research undertaken and there are no financial implications.
References
Ballot, A., Kotut, K., Novelo, E., & Krienitz, L. (2009). Changes of phytoplankton communities in Lakes Naivasha and Oloidien, examples of degradation and salinization of lakes in the Kenyan Rift Valley. Hydrobiologia, 632, 359-363.
Indexed at, Google Scholar, Cross Ref
Bellinger, E. G., & Sigee, D. C. (2015). Freshwater algae: identification, enumeration and use as bioindicators. John Wiley & Sons.
Indexed at, Google Scholar, Cross Ref
Flöder, S., Jaschinski, S., Wells, G., & Burns, C. W. (2010). Dominance and compensatory growth in phytoplankton communities under salinity stress. Journal of Experimental Marine Biology and Ecology, 395(1-2), 223-231.
Indexed at, Google Scholar, Cross Ref
Hubble, D. S., & Harper, D. M. (2002). Phytoplankton community structure and succession in the water column of Lake Naivasha, Kenya: a shallow tropical lake. Hydrobiologia, 488, 89-98.
Kathiresan, M. (2013). Composition and community structure of plankton from muthupet coastal waters and application of marine copepod Oithona rigida for Larval rearining of pacific white shrimp Litopen Aeus Vannamei.
Larson, C. A., & Belovsky, G. E. (2013). Salinity and nutrients influence species richness and evenness of phytoplankton communities in microcosm experiments from Great Salt Lake, Utah, USA. Journal of plankton research, 35(5), 1154-1166.
Indexed at, Google Scholar, Cross Ref
Ndungu, J., Augustijn, D. C., Hulscher, S. J., Fulanda, B., Kitaka, N., & Mathooko, J. M. (2014). A multivariate analysis of water quality in Lake Naivasha, Kenya. Marine and freshwater research, 66(2), 177-186.
Nielsen, D. L., Brock, M. A., Rees, G. N., & Baldwin, D. S. (2003). Effects of increasing salinity on freshwater ecosystems in Australia. Australian Journal of Botany, 51(6), 655-665.
Indexed at, Google Scholar, Cross Ref
Nyangau, G. (2021). Assessment of fisheries changes in relation to water level fluctuations, Species introductions and management trend in the Lake Naivasha (Doctoral dissertation).
Olofsson, M., Hagan, J. G., Karlson, B., & Gamfeldt, L. (2020). Large seasonal and spatial variation in nano-and microphytoplankton diversity along a Baltic Sea—North Sea salinity gradient. Scientific Reports, 10(1), 17666.
Omondi, A. O., Balaka, S. O., Mokua, G. O., & Onchieku, J. (2020). A review of the changes in phytoplankton community structure and ecology in Lake Naivasha, Kenya.
OpenStreetMap.org, (2021). https://www.researchgate.net
Rayori, D., Getabu, A., Omondi, R., Orina, P., Gisacho, B., & Omondi, A. (2021). Phytoplankton diversity in Gusii wastewater treatment plant in Kisii County, Kenya. Int J Fish Aquat Stud, 9(3), 299-306.
Rochelle-Newall, E. J., Chu, V. T., Pringault, O., Amouroux, D., Arfi, R., Bettarel, Y., ... & Torréton, J. P. (2011). Phytoplankton diversity and productivity in a highly turbid, tropical coastal system (Bach Dang Estuary, Vietnam). Biogeosciences Discussions, 8(1), 487-525.
Roy, K., Gupta, S., & Nandy, S. K. (2016). Checklist of commonly occurring phytoplankton and zooplankton genera of urban and rural ponds of raipur, chhattisgarh. International Journal of Research in Biological Sciences, 6(1), 1-6.
Strigo, I. A., & Craig, A. D. (2016). Interoception, homeostatic emotions and sympathovagal balance. Philosophical Transactions of the Royal Society B: Biological Sciences, 371(1708), 20160010.
Indexed at, Google Scholar, Cross Ref
Suthers, I., Rissik, D., & Richardson, A. (2019). Plankton: A guide to their ecology and monitoring for water quality. CSIRO publishing.
Telesh, I. V., & Khlebovich, V. V. (2010). Principal processes within the estuarine salinity gradient: a review. Marine Pollution Bulletin, 61(4-6), 149-155.
Indexed at, Google Scholar, Cross Ref
Velasco, J., Gutiérrez-Cánovas, C., Botella-Cruz, M., Sánchez-Fernández, D., Arribas, P., Carbonell, J. A., ... & Pallarés, S. (2019). Effects of salinity changes on aquatic organisms in a multiple stressor context. Philosophical Transactions of the Royal Society B, 374(1764), 20180011.
Indexed at, Google Scholar, Cross Ref
Received: 26-Sep-2023, Manuscript No. JMIDS-23-14034; Editor assigned: 27-Sep-2023, Pre QC No. JMIDS-23-14034(PQ); Reviewed: 11-Oct-2023, QC No. JMIDS-23-14034; Revised:13-Oct-2023, Manuscript No. JMIDS-23-14034(R); Published: 24-Oct-2023