Research Article: 2024 Vol: 27 Issue: 1
Variation in Water Quality Variables and the Zooplankton along A Salinity in the Lakes Oloiden and Naivasha
Guto Kerubo Carolyne, Kisii University
Njiru Murithi James, Kisii University
Getabu Albert, Kisii University
Gichana Moraa Zipporah, Kisii University
Citation Information: Kerubo Carolyne, G., Murithi James, N., Albert, G., & Moraa Zipporah, G. (2024). Variation in water quality variables and the zooplankton along a salinity in the lakes oloiden and naivasha. Journal of Management Information and Decision Sciences, 27 (S1), 1-12.
Abstract
The water level increased in the Lake Naivasha leading to its merging with Lake Oloiden forming an estuary. A study was done monthly, for one year in the Lakes Naivasha and Oloiden: with an objective to investigate the variation in water quality variables and their effect on zooplankton along a salinity gradient in the Lakes Oloiden and Naivasha. Water quality variables were measured in situ and the zooplankton sampled in each of the sites. The Lake Oloiden had higher total dissolved solids (0.41±0.085 mg/l), salinity (0.310±0.065 ppt), temperature (21.54±2.19 o C)), conductivity (0.58±0.1 μS/m) and pH (8.51±0.3) compared to Lake Naivasha. Temperature, pH, conductivity, total dissolved solids were lower than previous findings while dissolved oxygen was similar. Brachionidae had the highest number of species, however its density was lower as compared to Daphnidae, Sisidae and Cyclopoidae. Cyclopoidae had a negative correlation with the conductivity and total dissolved solids while salinity's correlation was positive ain Lake Naivasha: its correlation was positive with conductivity and total dissolved solids and negative with salinity and dissolved oxygen in Lake Oloiden. There was variation in the water quality variables and all families had associations with water quality variables in Lake Oloiden while only Lake Naivasha’s Cyclopoidae had. Further research should be done on zooplankton variation with depth post water level rise.
Keywords
Salinity; Salinity Gradient, Water Quality Variables; Zooplankton Species.
Introduction
Hydrological changes did occur in Lake Naivasha; water-level increased leading to its merging with Lake Oloiden (Ballot et al., 2009; Nyangau, 2021). The Lake Oloiden is alkaline-saline while Lake Naivasha is a fresh-water (Hubble & Harper, 2002). This phenomenon led to the creation of an estuarine environment. A salinity gradient is a distinguishing feature in an estuarine environment. Salinity is a major factor which defines the ecosystem and plays a critical role in defining the structure and functioning of aquatic biota (Telesh & Khelebivich, 2010).
The chemical and physical processes in an estuary vary non-linearly: its biological, physical and chemical features a may be distinguished by a high productivity. Zooplankton community are diverse and they occupy many intermediate trophic levels; they are sensitive to changes in the aquatic ecosystem they occur which makes them indicators (Suther et al., 2019). The ecosystem conditions dictate the zooplankton type, structure, population and the productivity in the pelagic (Telesh & Khelebivich, 2010). Although, zooplankton species diversity may also be defined by biotic components e.g., competition, predation (Telesh & Khelebivich, 2010; Mironga et al., 2014).
In the Neva estuary (riverine fresh water and saline water mix) the zooplankton community structure varied on regularity. At instance where there was an increase in the salinity; the rotifer biomass decreased to 10% (0.37 PSU) and was 45% (3.85 PSU) and some euryhaline species were favored e.g., Keratella cochlearis baltica. There was a variation in the number of species. At salinities >3 PSU the dominant fresh water copepods: Mesocyclop sp. and Thermocyclop sp. were largely replaced by brackish water species. The species composition in relation to cladocera varied insignificantly; the biomass reduced with the increase in the salinity (Telesh & Khelebivich, 2010). Salinity is an important environmental factor that may shape the biodiversity and richness of zooplankton. Investigation along the salinity gradient may provide information that is key in understanding zooplankton distribution (Yuan et al., 2020). The dissolved oxygen was a key factor that affected the distribution of zooplankton species in Lake Naivasha: sites with Pontederia crassipes had low dissolved oxygen and a lower number of zooplankton species (Mironga et al., 2014). Little is known about the variation various water quality parameters and their effect on the zooplankton community along the salinity gradient in Lakes Oloiden and Naivasha. Thus, the objective of the study in investigating the variation in water quality variables and their effect on zooplankton along a salinity gradient in the Lakes Oloiden and Naivasha (Mavuti & Harper, 2006).
Materials and Methods
Study Site
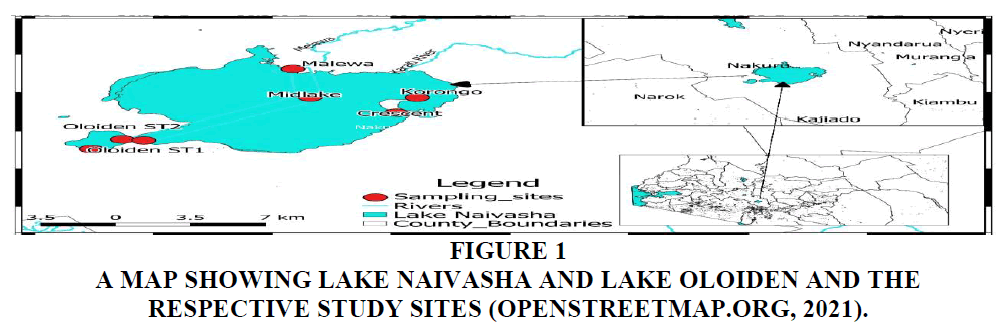
The study was done monthly, for one year in the Lake Oloiden (00°50’S, 36°17’E) and Lake Naivasha (00o46’S, 36o22’E) (Ballot et al., 2009). A transect was drawn from Oloiden ST1 to Malewa and two additional sites in Lake Naivasha were added in the sampling. The study siyqaes were: Oloiden ST1, Oloiden ST2, Oseria, Midlake, Malewa, Crescent (a crater lake connected during high water levels) and Korongo (a lagoon that’s connected to Lake Naivasha). The Oloiden ST2 and Oseria were connected due to the high-water levels. Oseria had its shoreline covered by a light layer of Cyperus papyrus and Pontederia crassipes mat and their density increased depending on the wind direction. The Lake Naivasha study sites also had these plants at the edge: with exception of Midlake (open water) Figure 1.
Figure 1 A Map Showing Lake Naivasha and Lake Oloiden and the respective study sites (Openstreetmap.org, 2021).
Water Quality
The water quality parameters were measured in situ using a YSI Multiparameter meter in each site: temperature, dissolved oxygen, conductivity, total dissolved solids, salinity and pH.
Zooplankton Sampling
A zooplankton net (60 μm) with a flow meter at the mouth was towed for two trips at 1-2m/s: where readings were recorded prior and after towing (aided in the calculation of the volume of water filtered through). Thereafter, the sample obtained was preserved (adding drops of 4% formalin). In the laboratory, the sample was measured and a subsample of 1ml was diluted in 10ml of distilled water. Counting was done using a Sedgwick Rafter cell (paralel scanningq), under a compound microscope (×100) with aid of identification keys (Sarma & Elías-Gutiérrez, 1999; Suther et al., 2019; Dang et al., 2015). The volume of water that was sieved through zooplankton net was calculated using the standard formulae as stated in EPA GLNPO, 2016.
Statistical Analysis
One-way Analysis of Variance (ANOVA, 95% CI) was utilized in checking the variations in water quality variables; while Pearson’s correlation was done between water quality and zooplankton (One tailed, 95% and 99% CI). ANOVA and Pearson’s correlation were done in Statistical Package for Social Scientists (SPSS) while the zooplankton density was calculated in R (version 4.3.1).
Results
Water Quality
The temperature was significantly different between the study sites and lakes (P<0.05) and Lake Oloiden’s was higher than Lake Naivasha (Table 1). Dissolved oxygen (DO) had significantly differences (P<0.05) between the study site; Korongo (5.85 ± 1.46 mg/l) had the lowest and Midlake (6.82 ± 1.66 mg/l) had the highest. Lake Oloiden's dissolved oxygen was similar to Lake Naivasha's. The study sites and lakes’ conductivity were significantly different (P<0.05) and Lake Naivasha’s was lower compared to Lake Oloiden’s. Total dissolved solids (TDS) were significantly different (P<0.05) amongst study sites and lakes. A significant difference in the study sites and lakes’ salinity was noted (P<0.05) and Lake Oloiden’s was higher as compared to Lake Naivasha; Oloiden ST2 (0.312 ppt) had the highest. The difference between the study sites and lakes’ pH were significant (P<0.05): Lake Oloiden’s was higher than Lake Naivasha’s (OpenStreetMap, 2021).
| Table 1 Water Quality in the Various Sites in Lake Oloiden and Lake Naivasha | |||||||
| Oloiden ST1 | Oloiden ST2 | Oseria | Midlake | Malewa | Crescent | Korongo | |
| TemperatureoC | 21.96±1.12 | 21.12±3.25 | 20.8±2.8 | 20.08±3.84 | 21.97±1.23 | 21.39±0.67 | 21.74±1 |
| DO (mg l-1) | 6.29±2.2 | 6.57±1.3 | 5.95±1 | 6.73±1.3 | 6.54±0.8 | 5.87±0.9 | 5.88±0.7 |
| Conductivity (µS m-1) | 0.58±0.1 | 0.58±0.1 | 0.19±0.04 | 0.18±0.04 | 0.18±0.04 | 0.17±0.04 | 0.19±0.4 |
| Salinity (ppt) | 0.31±0.07 | 0.31±0.06 | 0.098±0.02 | 0.096±0.02 | 0.093±0.02 | 0.096±0.02 | 0.098±0.02 |
| pH | 8.56±0.2 | 8.46±0.4 | 7.68±0.4 | 7.65±0.2 | 7.52±0.2 | 7.45±0.3 | 7.62±0.2 |
Zooplankton Density
Branchionidae had the highest number of zooplankton species: Brachionus falcatus (Oloiden ST2) had the highest abundance, Brachionus plicatilis was only present in Oloiden ST1 and ST2 while Brachionus forficula was only present in Malewa. Brachionidae had a higher density in Oloiden ST1 and ST2 as compared to the rest of sites while Trichocercidae density that was lower.
Moina macropa had the highest abundance followed by Thermocyclop crassus, Mesocyclop sp. then Diaphanosoma sarsi. Lecane lunaris was only in Oseria similar to Plationus patulus in the Midlake and their density was not high. Trichocercidae, Lecanidae and Brachionidae had a low abundance as compared to the rest of the families (Daphnidae, Sisidae and Cyclopoidae) (Table 2).
| Table 2 The Zooplankton Density (Individuals Per Litre) in the Respective Study Sites in Lake Oloiden and Lake Naivasha. | |||||||
| Oloiden ST1 | Oloiden ST2 | Oseria | Crescent | Korongo | Midlake | Malewa | |
| Brachionidae | |||||||
| Brachionus caudata | 7,934 | 11,035 | 3,348 | 15,492 | 6,283 | 15,603 | |
| Brachionus angularis | 26,336 | 1,759 | 3,394 | ||||
| Brachionus calyciflorus | 3,376 | 1,759 | 6,283 | ||||
| Brachionus diversicornis | 2,701 | 3,518 | 7,935 | 5,654 | 3,394 | 3,601 | |
| Brachionus forficula | 3,601 | ||||||
| Brachionus plicatilis | 8,103 | 12,314 | |||||
| Brachionus falcatus | 34,033 | 85,319 | 3,637 | 3,665 | 5,656 | 7,201 | |
| Brachionus quadridentalus | 2,976 | 3,394 | |||||
| Plationus patulus patulus | 6,788 | ||||||
| Lecanidae | |||||||
| Lecane lunaris | 2,975 | ||||||
| Trichocercidae | |||||||
| Trichocerca similis | 2,025 | 1,759 | 7,439 | 3,394 | 3,601 | ||
| Sididae | |||||||
| Diaphanosoma Sarsi | 36,212 | 32,691 | 64,436 | 57,205 | 53,544 | 117,000 | 83,116 |
| Daphnidae | |||||||
| Moina macropa | 121,971 | 97,633 | 191,053 | 219,193 | 185,468 | 227957 | 234,945 |
| Cyclopoidae | |||||||
| Tropocyclop prasinus prasinus | 3,376 | 4,691 | 8,802 | 5477 | 14,136 | 63,070 | 10,442 |
| Microcyclops varicans varicas | 11,902 | 11,619 | 14,136 | 81,454 | 18,003 | ||
| Mesocyclop p. | 57,736 | 42,132 | 73,581 | 86,185 | 83,768 | 87,007 | 116,873 |
| Thermocyclops crassus | 58,933 | 50,856 | 105,772 | 126,623 | 135,547 | 918,324 | 145,228 |
Branchionidae was present in all study sites except the Cresent; its density was low as compared to Daphnidae, Sisidae and Cyclopoidae (Table 3).
| Table 3 Zooplankton Density of the Various Families in the Respective Study Sites of Lakes Naivasha and Oloiden | |||||||
| Taxonomic group | Oseria | Crescent | Korongo | Midlake | Malwa | Oloiden ST1 | Oloiden ST2 |
| Brachionidae | 1,988 | - | 2,736 | 2,514 | 3,751 | 10,311 | 14,463 |
| Lecanidae | 2,976 | - | - | - | - | - | - |
| Trichocercidae | - | 7,439 | 3,394 | 3,601 | - | 2,026 | 1,759 |
| Sisidae | 57,205 | 64,436 | 117,000 | 3,116 | 53,544 | 36,212 | 32,691 |
| Daphnidae | 219,194 | 191,052 | 227,957 | 227,957 | 185,468 | 121,971 | 97,633 |
| Cyclopoidae | 57,476 | 50,015 | 80,839 | 80,839 | 61,897 | 30,011 | 24.420 |
Correlation amongst water Quality Parameters and Zooplankton Families
Brachionidae negatively correlated with the conductivity, temperature, total dissolved solids, salinity and pH while it positively correlated with the dissolved oxygen in the Lake Oloiden (Table 4). Trichocercidae, Sisidae and Daphnidae had a positive correlation with all the water quality variables except for the dissolved oxygen. Cyclopoidae positively correlated with the TDS and conductivity while salinity and dissolved oxygen’s was negative. Brachionidae negatively correlated with Trichocercidae, Sisidae and Cyclopoidae but positively correlated with Daphnidae. Trichocercidae positively correlated with Cyclopoidae, Daphnidae, Sisidae while it negatively correlated with Brachionidae. Sisidae and Cyclopoidae negatively corrrelated with Brachionidae; positively correlated with Trichocercidae while the earlier also positively correlated with Cyclopoidae and later also positively correlated with Daphnidae and Sisidae. Daphnidae had a positive correlation with all the families.
| Table 4 Pearson Correlation of Zooplankton Families and Some Water Quality Variables in Lake Oloiden (Temp =Temperature and, Cond=Conductivity ((P=0.01)**, (P=0.05)*) | |||||||||||
| Branchionidae | Trichocercidae | Sisidae | Daphnidae | Cyclopoidae | Temp | DO | Cond | TDS | Salinity | pH | |
| Brachionidae | 1 | -1** | -1** | 1** | -1** | -1** | 1** | -1** | -1** | -1* | -1** |
| Trichocercidae | -1** | 1 | 1** | 1** | 1** | 1** | -1** | 1** | 1** | 1** | 1** |
| Sisidae | -1** | 1** | 1 | 1** | 1** | 1** | -1** | 1** | 1** | 1** | 1** |
| Daphnidae | -1** | 1** | 1** | 1 | 1** | 1** | -1** | 1** | 1** | 1** | 1** |
| Cyclopoidae | -1** | 1** | 1** | 1** | 1 | 0 | 0 | -0.71* | -0.08* | -0.09** | 0 |
| Temp (o C) | -1** | 1** | 1** | 1** | 0 | 1 | 0.26** | 0.14** | 0.1** | 0.3 | 0.29** |
| DO | 1** | -1** | -1** | -1** | -1** | 0.26** | 1 | -0.33** | -0.35** | -0.38** | 0.15** |
| Cond (µS/m) | -1** | 1** | 1** | 1** | 1** | 0.14** | -0.33** | 1 | 0.93** | 0.95** | 0.17** |
| TDS (mg/l) | -1** | 1** | 1** | 1** | 0.08** | 0.1** | -0.35** | 0.93** | 1 | 0.93** | 0.14** |
| Salinity (ppt) | 1** | 1** | 1** | 1** | -0.09** | 0 | -0.38** | 0.95** | 0.93** | 1 | 0.15** |
| pH | -1** | 1** | 1** | 1** | 0 | 0.29** | 0.15** | 0.17** | 0.14** | 0.15** | 1 |
The temperature had a positive correlation with the dissolved oxygen, conductivity, total dissolved solids and pH. Dissolved oxygen positively correlated with temperature and pH and negatively correlated with the conductivity, total dissolved solids and salinity. The conductivity and TDS had a positive correlation with the temperature pH, TDS and salinity while the dissolved oxygen’s correlation was negative. There was a negative correlation of salinity with the dissolved oxygen and a positive correlation with pH, conductivity and TDS. pH’s correlation with all the variables was positive.
Cyclopoidae had a negative correlation with the conductivity and total dissolved solids while salinity’s was positive in the Lake Naivasha (Table 5). Brachionidae negatively correlated with Trichocercidae. Trichocercidae’s correlation with Daphnidae and Brachionidae was negative. Daphnidae negatively correlated with Trichocercidae. The temperature positively correlated with the dissolved oxygen and TDS while it negatively correlated with the salinity. Dissolved oxygen’s correlation was negative with the conductivity, TDS and salinity while temperature and pH’s was positive. The conductivity positively correlated with the TDS, pH and salinity while the dissolved oxygen’s correlation was negative. Total dissolved solids had a positive correlation with the dissolved oxygen, conductivity, salinity, pH while temperature’s was negative. Salinity had a negative correlation with the temperature and a positive correlation with the conductivity and TDS. The pH’s correlation was positive with the temperature, dissolved oxygen, conductivity and TDS.
| Table 5 Pearson Correlation of Zooplankton Families and Some Water Quality Variables in Lake Naivasha ((p=0.01)**, (p=0.05)* and a (Couldn’t be Calculated because at least one Variable was Constant)). | ||||||||||||
| Branchionidae | Lecanidae | Trichocercidae | Sisidae | Daphnidae | Cyclopoidae | Temp | DO | Cond | TDS | Salinity | pH | |
| Brachionidae | 1 | a | -1** | 0.1 | -0.8 | -0.19 | -0.71 | -0.76 | -0.79 | -0.83 | -0.83 | -0.86 |
| Lecanidae | a | 1 | a | a | a | a | a | a | a | a | a | a |
| Trichocercidae | -1** | a | 1 | -0 | -1* | -0.64 | 0.99 | 0.91 | 0.16 | -0.41 | -0.46 | -0.99 |
| Sisidae | 0.1 | a | -0 | 1 | 0.02 | -0.65 | 0.12 | 0.31 | -0.35 | -0.51 | -0.41 | -0.14 |
| Daphnidae | -1 | a | -1* | 0.1 | 1 | 0.38 | 0.28 | 0.4 | 0.41 | 0.49 | 0.43 | 0.26 |
| Cyclopoidae | -0 | a | -1 | -1 | 0.59 | 1 | -0.05 | 0.03 | -0.1** | -0.09** | 0.1** | 0.02 |
| Temp (o C) | -1 | a | 1 | 0.1 | -0.2 | -0.05 | 1 | 0.2** | 0.04 | 0.08** | -0.14** | 0.2 |
| DO | -1 | a | 0.9 | 0.3 | -0.1 | 0.03 | 0.2** | 1 | 0.33** | -0.36** | -0.3** | 0.3** |
| Cond (µS/m) | -1 | a | 0.2 | -0 | 0.31 | -0.1** | 0.04 | -0.33** | 1 | 0.86** | 0.83** | 0.09** |
| TDS (mg/l) | -1 | a | -0 | -1 | 0.49 | -0.09** | -0.08** | 0.36** | 0.86** | 1 | 0.75** | 0.13** |
| Salinity (ppt) | -1 | a | -0 | -4 | 0.48 | -0.1** | -0.14** | -0.3** | 0.83** | 0.75** | 1 | -0.02 |
| pH | -1 | a | -1 | -0 | 0.62 | 0.02 | 0.2** | 0.3** | 0.09** | 0.13** | -0.19 | 1 |
Discussion
The Oloiden ST1 and ST2 (Lake Oloiden’s) temperature, conductivity, total dissolved solids, salinity and pH were higher as compared to Lake Naivasha (Hubble & Harper, 2002; Ballot et al., 2009). Temperature was as per the previous findings in Lake Naivasha (Ndungu et al., 2014). pH, conductivity, total dissolved solids and dissolved oxygen was lower than the previous findings (Ballot et al., 2009) Salinization may have occurred in the Lake Naivasha due to its merging with Lake Oloiden (Nielsen et al., 2003). All changes in the salinity, water level, temperature may have an influence on residential and ecological community (D’alelio et al., 2022). The temperature increased with the dissolved oxygen, conductivity, total dissolved solids and pH increase in Lake Oloiden increase. Dissolved oxygen increased with the temperature and pH and decreased with increase in the conductivity, TDS and salinity increase. A rise in the temperature allows phytoplankton to photosynthesize; producing a by-product of oxygen (led to increased DO) and hydroxide ions (Kathiresan, 2013). The increase in hydroxide ions led to an increase in pH and total dissolved solids: which in turn led to increase in the conductivity. Dissolved oxygen rose with temperature and pH increase; while it decreased with the conductivity, total dissolved solids and salinity (Suthers et al., 2019).
The conductivity and TDS increased simultaneous with the temperature pH, TDS and salinity while it decreased with dissolved oxygen’s increase. An increase in ions led to more electrical conductivity; which increased the temperature, pH, TDS and salinity (Nielsen et al., 2003). The Oloiden ST2 had the highest conductivity, TDS and salinity; which could be due to increased ionic content based on the complexity of biogeochemical processes taking place in an estuary (Telesh & Khelebivich, 2010). Salinity decreased with the dissolved oxygen increase and simultaneously rose with pH, conductivity and TDS. Decomposition of organic matter may have taken place and thus the production of hydrogen ions; increasing the pH, conductivity and total dissolved solids (Ndungu et al., 2014). pH increased with all the variables increase: hydroxide and hydrogen ions affect the pH; increment in ions allows more electric ion conductivity which may led to increase in other variables i.e., temperature, dissolved oxygen, conductivity, TDS and salinity (Nielsen et al., 2003).
The temperature rose as dissolved oxygen, TDS and pH increased while it decreased with the salinity increase in the Lake Naivasha. Once the temperature increased there was photosynthesis that took place which produced oxygen and hydroxide ions; leading to an increase in pH and TDS (Kathiresan, 2013). The dissolved salt in the water contribute to the salinity; increment in ionic component affects it (Nielsen et al., 2003). Dissolved oxygen decreased as the conductivity, TDS and salinity rose: increased simultaneous with the temperature and pH. A decrease in dissolved oxygen could be due to decomposition of organic matter; comes in through discharge from the catchment and detritus from acacia trees (Vachellia xanthophloea) that fell into the water post water level rise. The Midlake had the highest dissolved oxygen while Korongo’s was the lowest: the open water site had no fall of trees in the water as compared to Korongo; an edge site. The high detritus may have contributed to the lower dissolved oxygen in Lake Naivasha than previous (Ndungu et al., 2014). Increased conductivity, TDS and salinity could be due do production of hydrogen ions during respiration; increasing ionic content (Ndungu et al., 2014); in turn affected the amount of salt that was dissolved (Kathiresan, 2013).
Conductivity increased simultaneous with the TDS, pH and salinity while contrasting with dissolved oxygen. Increased conductivity was due to increase in ions: inorganic material and dissolved salt; contributing towards increase in pH, TDs and salt respectively (Nielsen et al., 2003). The total dissolved solids increased with temperature, conductivity, salinity and pH increase while it decreased with dissolved oxygen’s increase; could be due to photosynthesis then respiration that took place (Kathiresan, 2013). pH increased with the temperature, dissolved oxygen, conductivity and TDS increase. The PH in Lake Naivasha was lower than its previous (Ballot et al., 2009; Ndungu et al., 2014). Its increment could be due to temperature rise: phosynthetic organisms utilize it and produce more dissolved oxygen and hydroxide ions; contributing to the increment in TDS then conductivity.
Seventeen zooplankton species were identified in Lake Naivasha; which was line with previous findings in L. Naivasha (Mironga et al., 2014) and Lake Oloiden’s were 12 species. The changes in an ecosystem may be noted by the species composition and density; there were differences in species composition in Lake Naivasha (Kathiresa, 2013; Mironga et al., 2014). All the species that belonged to Brachionidae were present in Oloiden ST1 and ST2 with exception of Brachionus quadridentatus quadridentatus, Plationus patulus patulus and Branchionus forficula. Brachionus caudata’s density was highest in Crescent, followed by Malewa and Oloiden ST2. Oseria’s abundance was the lowest as compared to Oloiden ST2; showing non-linear variation in an estuarine condition (Telesh & Khelebivich, 2010).
Brachionus angularis was only present in Oloiden ST1, ST2 and the Midlake; Oloiden ST1 had the highest density while Oloiden ST2 had the lowest. There may be attributed to variations caused by salinity (Telesh & Khelebivich, 2010). Brachionus calyciflorus was present in Oloiden ST1, ST2 and Korongo (had the highest density). Korongo was a lagoon; site prevailing conditions may affect abundance (Mironga et al., 2014). Brachionus diversicornis was present in all sites except Crescent: Oseria had the highest density while Oloiden ST2’s was less than its half yet both were estuarine; there may be higher productivity and non-linearity (Telesh & Khelebivich, 2010).
Brachionus forficula was only present in Malewa while Plationus patulus patulus was only present in the Midlake. Brachionus plicatilis was only present in Lake Oloiden and Oloiden ST2’s was higher; could be due to its estuarine nature. It had high temperature; which my favor high primary productivity and cascading to zooplankton. Brachionidae decreased with increase in the conductivity, temperature, total dissolved solids, salinity and pH while it increased with the dissolved oxygen in Lake Oloiden. which was line with previous findings in L. Naivasha (Mironga et al., 2014). That was confirmed by density of Brachionus falcatus (Yuan et al., 2020). although, there were variations prevailing site conditions may affect biodiversity (Mironga et al., Trichocercidae, Sisidae and Daphnidae density increased with the increase in all water quality variables except the dissolved oxygen in the Lake Oloiden. On the contrary the only specie belonging to Sisidae and Daphnidae had the highest density in the open water site (Midlake); which had the highest dissolved oxygen tallying with Mironga et al. (2014). Cyclopoidae density increased with the TDS and conductivity increase; decreased with dissolved oxygen and salinity increase. The density of all its species decreased with the increased salinity. Environmental factors may impact the zooplankton community (Telesh & Khelebivich, 2010).
Cyclopoidae: Tropocyclop prasinus prasinus was present in all the study sites and the Midlake’s had the highest abundance. It was followed by Korongo, Malewa then Oseria; Oloiden ST2’s density was almost half of Oseria; variations in salinity may have an effect on the density (Yuan et al., 2020). Microcyclops varicans varicans was present only in Lake Naivasha and the Midlake’s abundance was the highest, which could be due to its high dissolved oxygen. All the other sites in the Lake Naivasha had some Cyperus papyrus and Pontederia crassipes which may contribute to the reduction of DO (Mironga et al., 2014).
Mesocyclop sp abundance was high: although Lake Oloiden sites were lower than Lake Naivasha’s; salinity may affect biodiversity. Malewa had the highest density while Oseria’s was higher as compared to Oloiden ST2; non-linear variation in an estuarine ecosystem (Telesh & Khelebivich, 2010) and variations in salinity (Yuan et al., 2020). Thermocyclops crassus also had the highest abundance in Lake Naivasha; more than twice Lake Oloiden’s; prevailing environmental variables may have favored. Consequently, Oseria’s density was twice Oloiden ST2’s. Thus, there is high productivity in an estuary; although there was non-linearity was exhibited (Telesh & Khelebivich, 2010).
There was high variability in the Daphnidae specie density: its highest was in the Midlake, followed by Malewa then Oseria while Oloiden ST2’s was the lowest. The Midlake had the highest dissolved oxygen; thus, site conditions may affect abundance and distribution (Mironga et al., 2014). Moina macropa (Daphnidae) was present in all the sites while Lecane lunaris (Lecanidae) was present in Oseria only; the earlier family may be used as an indicator of ecosystem health since its more tolerant to variations. All the Cyclopoidae species were present in all sites except Microcyclops varicans varicans (Oloiden ST1 and ST2). Salinity may be an influencing factor that may limit the plankton community.
The Brachionidae decreased with the increase in Trichocercidae, Sisidae, Daphnidae and Cyclopoidae in Lake Oloiden. On contrary was the abundance of Trichocercidae; salinity may have a negative effect on rotifer abundance. Density results conquered with Cyclopoidae, Daphnidae, Sisidae (Yuan et al., 2020). Sisidae and Cyclopoidae decreased with increase in Branchionidae and increased concurrent with Trichocercidae, while earlier increased with Cyclopoidae increase and later also increases increase Daphnidae and Sisidae. Density results conquered for Brachionidae for both and Daphnidae for Cyclopoidae; may be well adapted for a saline environment (Yuan et al., 2020). Daphnidae increased as all the families did increase: its density was the highest; although contrary to previous findings in a saline environment (Velasco et al., 2019). Structural characteristics in the zooplankton community may show unevenness with changes in salinity (Yuan et al., 2020).
Cyclopoidae density decreased with the increase in the conductivity and total dissolved solids while it increased with increase salinity in Lake Naivasha. The Crescent’s had the lowest abundance and its TDS was characteristic; ions influenced by crater rocks (Guto et al., 2022). A Brachionidae decreased with increase in Trichocercidae: density decreased in Korongo and the Midlake. Biodiversity may have direct relation to stability and resilience to changes that may occur (Gain et al., 2008). Trichocercidae’s decreased with Daphnidae and Brachionidae increase: in line with density of Daphnidae. Daphnidae density increased with Trichocercidae’s increase; density results contrasted. Changes may occur at organismal level and finally affecting the zooplankton community (Velasco et al., 2019).
Conclusion
Temperature, pH, conductivity, total dissolved solids were lower than previous findings while dissolved oxygen was similar. Brachionidae had the highest number of species, however its density was low as compared to Daphnidae, Sisidae and Cyclopoidae. Cyclopoidae had a negative correlation with the conductivity and total dissolved solids while salinity had a positive correlation in Lake Naivasha: its correlation was positive with conductivity and total dissolved solids and negative with salinity and dissolved oxygen in Lake Oloiden. There was variation in the water quality variables and all families had associations with water quality variables in Lake Oloiden while only Lake Naivasha’s Cyclopoidae had. Further research should be done on zooplankton variation with depth post water level rise.
Acknowledgement
This study was supported by Kenya Marine and Fisheries Research Institute (KMFRI), Naivasha station, to the staff that aided in the acquiring of the samples, laboratory space and equipment; our gratitude.
References
Ballot, A., Kotut, K., Novelo, E., & Krienitz, L. (2009). Changes of phytoplankton communities in Lakes Naivasha and Oloidien, examples of degradation and salinization of lakes in the Kenyan Rift Valley. Hydrobiologia, 632, 359-363.
Indexed at, Google Scholar, Cross Ref
D’Alelio, D., Russo, L., Del Gaizo, G., & Caputi, L. (2022). Plankton under pressure: how water conditions alter the phytoplankton–zooplankton link in coastal lagoons. Water, 14(6), 974.
Indexed at, Google Scholar, Cross Ref
Dang, P., Van Khoi, N., Nguyet Nga, L. T., Thanh, D. N., & Ho Thanh, H. (2015). Identification handbook of freshwater zooplankton of the Mekong River and its tributaries. Mekong River Commission, Vientiane.
Gain, A. K., Uddin, M. N., & Sana, P. (2008). Impact of river salinity on fish diversity in the south-west coastal region of Bangladesh. Int. J. Ecol. Environ. Sci, 34(1), 49-54.
Guto, C. K., James, N. M., Albert, G., & Zipporah, G. M. (2022). Spatial and temporal variation in water quality between the Lakes Naivasha and Oloiden in the Kenyan Rift Valley.
Hubble, D. S., & Harper, D. M. (2002). Phytoplankton community structure and succession in the water column of Lake Naivasha, Kenya: a shallow tropical lake. Hydrobiologia, 488, 89-98.
Kathiresan, M. (2013). Composition and community structure of plankton from muthupet coastal waters and application of marine copepod Oithona rigida for Larval rearining of pacific white shrimp Litopen Aeus Vannamei.
Mavuti, K. M., & Harper, D. (2006). The ecological state of Lake Naivasha, Kenya, 2005: Turning 25 years research into an effective Ramsar monitoring programme.
Mironga, J. M., Mathooko, J. M., & Onywere, S. M. (2014). Effects of spreading patterns of water hyacinth (Eichhornia crassipes) on zooplankton population in Lake Naivasha, Kenya. International Journal of Development and Sustainability, 3(10), 1971-1987.
Ndungu, J., Augustijn, D. C., Hulscher, S. J., Fulanda, B., Kitaka, N., & Mathooko, J. M. (2014). A multivariate analysis of water quality in Lake Naivasha, Kenya. Marine and freshwater research, 66(2), 177-186.
Indexed at, Google Scholar, Cross Ref
Nielsen, D. L., Brock, M. A., Rees, G. N., & Baldwin, D. S. (2003). Effects of increasing salinity on freshwater ecosystems in Australia. Australian Journal of Botany, 51(6), 655-665.
Indexed at, Google Scholar, Cross Ref
Nyangau, G. (2021). Assessment of fisheries changes in relation to water level fluctuations, Species introductions and management trend in the Lake Naivasha (Doctoral dissertation).
OpenStreetMap.(2021). OpenStreetMap Nominatim. https://www.researchgate.net
Sarma, S.S.S., & Elías-Gutiérrez, M. (1999). Rotifers (Rotifera) from four natural water bodies of Central Mexico. Limnologica, 29(4), 475-483.
Indexed at, Google Scholar, Cross Ref
Suthers, I., Rissik, D., & Richardson, A. (2019). Plankton: A guide to their ecology and monitoring for water quality. CSIRO publishing.
Telesh, I. V., & Khlebovich, V. V. (2010). Principal processes within the estuarine salinity gradient: a review. Marine Pollution Bulletin, 61(4-6), 149-155.
Indexed at, Google Scholar, Cross Ref
Velasco, J., Gutiérrez-Cánovas, C., Botella-Cruz, M., Sánchez-Fernández, D., Arribas, P., Carbonell, J. A., ... & Pallarés, S. (2019). Effects of salinity changes on aquatic organisms in a multiple stressor context. Philosophical Transactions of the Royal Society B, 374(1764), 20180011.
Indexed at, Google Scholar, Cross Ref
Yuan, D., Chen, L., Luan, L., Wang, Q., & Yang, Y. (2020). Effect of salinity on the zooplankton community in the Pearl River estuary. Journal of Ocean University of China, 19, 1389-1398.
Indexed at, Google Scholar, Cross Ref
Received: 25-Sep-2023, Manuscript No. JMIDS-23-14033; Editor assigned: 29-Sep-2023, Pre QC No. JMIDS-23-14033(PQ); Reviewed: 18- Oct-2023, QC No. JMIDS-23-14033; Revised: 22-Oct-2023, Manuscript No. JMIDS-23-14033(R); Published: 30-Oct-2023