Research Article: 2021 Vol: 24 Issue: 1S
Made to Order Baby? Ethical and Legal Dimensions of Three Parent Baby and Germline Therapy
Mahesh Deshpande, Deemed University
Shashikala Gurpur, Deemed University
Abstract
The three-parent baby concept turned into reality in April 2016 when a boy was conceived and born after his mother underwent a procedure known as Mitochondrial Replacement Therapy (MRT) by a fertility clinic in New York City (Zhang et al., 2017). The technique involved removing the defective mitochondrial DNA of mother’s egg and putting in its place a normal one from the egg of a donor and then fertilizing this altered egg with father’s sperm. The technique is not completely risk free. Although UK has regulations dealing with it in place since 2015 (The Human Fertilisation and Embryology (Mitochondrial Donation) Regulations 2015); in USA the FDA has not approved the procedure. The fertility clinic then decided to carry out the procedure in Mexico as it had lax rules with minimal supervision. Ukraine has permitted the use of this procedure-designed primarily to stop transmission of genetic disorders from the mother to the foetus- even for fertility treatment. Safety aside, two main issues arise for consideration, viz. ethicality and legality of such human assisted reproductive technique. It is argued that such procedure sets the human race on a ‘slippery slope’ of germ-line editing which might as well culminate in ‘made to order’ babies (Darnovsky, 2013). This article aims to explore the legal and ethical aspects of MRT, its sociocultural impact, and the possibility of having regulations in place in India to have effective oversight of the procedure before it’s used in India. A discussion on these issues among experts from diverse disciplines including Law, Biomedicine, Bioethics, and government agencies will benefit the society and ensure responsible research in human genetic engineering and assisted reproductive technology. Authors argue for a multidisciplinary discussion than a blanket ban on MRT or germline therapy.
Keywords
Three Parent Baby, Mitochondrial Replacement Therapy (MRT), Pronuclear Transfer (PNT), Maternal Spindle Transfer (MST), Mitochondrial DNA (MTDNA), Nuclear DNA (NDNA)
Introduction
The law of nature dictates that a human offspring inherit its set of genes from two persons of opposite sex i. e. a male and a female who are the father and the mother respectively. In this way all the genetic material, nuclear or mitochondrial, healthy or disease carrying, is transmitted to the child. Mitochondria are a cellular organelle providing energy for the cell’s functions. Located in the cytoplasm of the cell, they are also called its powerhouse. However, the mother alone, and not the father passes on the mitochondrial DNA (mtDNA) to the child. As such, a defective maternal mtDNA is passed on to all her offspring, male as well as female. Both sexes can be afflicted with the malady but only the females can transmit it to their children. The two most commonly occurring inherited mitochondrial diseases are Leigh Syndrome presenting as delayed psychomotor development, convulsions, abnormal ocular movements, vomiting, and respiratory abnormalities; and MELAS manifesting with myopathy, encephalopathy, lactic acidosis, and stroke-like episodes. However, severity of the disease depends on the load of the mutant or defective gene. Whether the disease manifests in the child or not depends on the amount of defective mitochondrial DNA, with some studies suggesting that the said amount necessary for clinical expression of the disease is as high as 60%. The idea of replacing the affected mitochondria of an embryo with a healthy one from another woman led to the term ‘three parent baby’ involving two women and a man. Such genetic alteration made to the gametes i. e. egg or sperm is called germline gene therapy because the altered DNA will be passed on to the descendants. The practice has generated controversy similar to that created by another genome modification technique called CRISPR/Cas9 (Clustered Regularly Interspaced Short Palindromic Repeats/Cas9).
Supporters of mitochondrial replacement therapy (MRT) define germline therapy as modification of nuclear DNA alone, and therefore claim that alteration of mtDNA is not germline therapy. Opponents, on the other hand, argue that such altered mtDNA may undergo additional mutations which the coming generations will inherit thereby making MRT a germline therapy. The three parent baby concept turned into reality in April 2016 when a boy was conceived and born after his mother underwent a procedure known as Mitochondrial Replacement Therapy (MRT) by a fertility clinic in New York City. The technique is not completely risk free. In USA the FDA has not approved the procedure. The fertility clinic then decided to carry out the procedure in Mexico as it had lax rules with minimal supervision. Dr. Zhang who carried out the procedure, revealed in an article published in April 2017, “The boy had neonatal mtDNA mutation load of 2.36–9.23% in his tested tissues. The boy is currently healthy at 7 months of age, although long-term follow-up of the child's longitudinal development remains crucial.” A certain apprehension runs among researchers that the faulty DNA will stage a comeback in the course of time. There is a distinct possibility that germline gene therapy will be used to produce babies with select characteristics (designer babies); an example of humans “playing God”. Already, MRT is being used to treat infertility, especially in elder women, which is a clinical application of the technique not originally intended. There is likelihood that MRT may also be used to produce genetically modified female embryos that will pass on the modifications to posterity altering the genome permanently.
Ukraine has permitted the use of this procedure, developed essentially to prevent transmission of genetic disorders from the mother to the foetus, even for fertility treatment. A boy was born in February 2017 after a girl was delivered in January, both of which are claimed to be healthy. Now there is a distinct possibility of transgene rational propagation of the girl’s modified genome when she reaches adulthood and has children of her own. Due to the unpredictability of its effects, the procedure is not risk free. Besides safety, number of ethical and legal issues spring to the fore. To tackle these, a robust and comprehensive regulatory frame work will be necessary. The U.K. is the only country which has taken the lead and passed a law (Human Fertilisation and Embryology Act 2008 (HFEA, 2008) and The Human Fertilisation and Embryology (Mitochondrial Donation) Regulations 2015) and constituted a statutory body to oversee its enforcement. The FDA in the US has so far steadfastly refused to accord approval for the procedure while Australia permits limited research in MRT on embryos not more than 14 days old, but at the same time bans its application in clinics. In India, MRT has not received much attention although it suggests a strong likelihood of its success. The prevalence rate of mitochondrial abnormality is 1 in 5000 with an individual mutation rate of 1 in more than 200 births. A strong desire to have a genetically connected healthy child which is not possible through adoption or surrogacy is likely to contribute to the acceptance and popularity of MRT in India in not too distant future. Ethical issues apart, MRT raises a plethora of legal conundrums. To what extent research into MRT and its clinical application should be legalised? What should constitute informed consent? Is mitochondrial donation an egg (gamete) donation or an organ donation? Should the donor identity be disclosed to the resulting child when it attains adulthood? How to and whom to assign parentage? Can the concerned parties mutually waive off parental rights and obligations? Will such waiver stand judicial scrutiny? Will the mitochondrial donor be liable for child support? What about succession (Child’s rights)?
In this article authors briefly look at the two distinct procedures of MRT viz. Maternal Spindle Transfer (MST) and Pronuclear Transfer (PNT). Next we focus our attention on the ethical dimensions of MRT, the harm it may cause to the child so conceived and to the egg donor, and its impact on the society. Thereafter we critically discuss the legal issues surrounding MRT and the necessity of regulatory framework to ensure responsible ethical research. Finally we conclude by observing that in case of genetic research, strict regulations are preferable to blanket ban and a multidisciplinary forum should be entrusted with the task of discussing and debating the complex ethical and legal issues surrounding MRT.
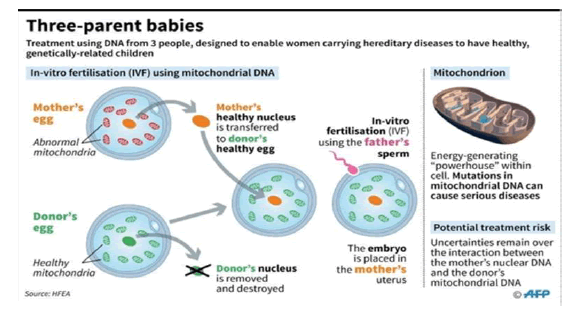
Mitochondrial Replacement Therapy (MRT) Procedures
The nucleus is removed from the donor’s ovum having normal mitochondria; nucleus is also removed from maternal ovum and placed inside the donor’s ovum which is then fertilised with the sperm of the father. The resulting embryo is transferred to the mother’s womb. Thus, two women contribute to the baby’s DNA: the mother giving the nuclear DNA (nDNA), and the donor the mitochondrial DNA (mtDNA).
The next two images explain in detail the procedure of maternal spindle transfer (MST) and pronuclear transfer (PNT) respectively.
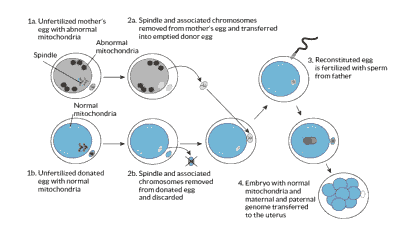
Maternal Spindle Transfer (MST)
“When a dividing cell divvies up its chromosomes, they are attached to protein fibers called microtubules or spindles. The transplant technique starts with two unfertilized egg cells, one from the donor and one from the mother. In both cells, the membrane surrounding the nucleus has broken down, but the cell has not yet completely divided. The spindle and its attached chromosomes are removed from the mother’s egg and inserted into the donor egg, which has been emptied of its spindle and chromosomes. Then a sperm cell is injected to the resulting egg to fertilize it.”
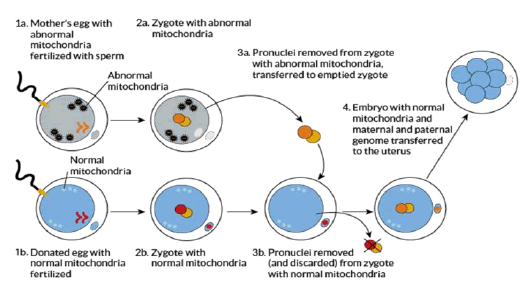
Pronuclear Transfer (PNT)
“Pronuclei are nuclei from the egg and sperm that are in the fertilized egg, called a zygote, but have not yet fused into a single nucleus. In this technique, the mother’s egg and a donor egg are fertilized at the same time. The pronuclei are removed from the donor egg and discarded. Then the pronuclei are sucked out of the mother’s egg and transferred into the empty donor egg” (Saey, 2016). The procedure involves destruction of two embryos in order to create a third one which also needs partial destruction, and raises serious questions as to ethics by exhibiting complete disregard for the sanctity of human embryos and treating them as dispensable spare parts and women as suppliers of those spares.
Additionally, as can be seen from above, both the procedures involve nuclear replacement and NOT mitochondrial replacement. The mitochondria in the donor egg are not touched at all whereas its nucleus is removed and substituted with the nucleus of the prospective mother’s egg. It is obvious that nuclear replacement is the actual procedure. In MST there is a distinct element of nuclear manipulation as well in both the eggs.
ETHICAL DIMENSIONS
Germline Modification
A controversial point is whether mtDNA alteration constitutes germline modification, as many scientists define it exclusively as modification of nuclear DNA. According to Stuart Newman, professor of cell biology and anatomy at New York Medical College,
“The procedure is not mitochondrial manipulation, it is nuclear manipulation amounting to genetic modification of human beings.” … “It was dangerous because it disrupted the evolutionary compatibility between the nucleus of a cell and the mitochondria of the cell. It is going to lead to children with conditions which, in some cases, will probably be worse than the conditions they are trying to avoid.”
But the Department of Health of Government of the UK says, “While there is no universally agreed definition (of ‘genetic modification’ in humans), the Government has decided to adopt a working definition for the purpose of taking forward these regulations. The working definition that we have adopted is that genetic modification involves the germ- line modification of nuclear DNA (in the chromosomes) that can be passed on to future generations. This will be kept under review.” This was challenged by prominent scientists who accused the government of lack of honesty, and, surreptitiousness.
A researcher states, “Mitochondrial replacement procedures would constitute germline modification”. Others seem to be more forthright in saying, “Mitochondrial gene replacement is especially challenging because the technique involves modification of the germline and modifications would be transmitted to subsequent generations. Reproductive research is unique in that although the patient undergoes the intervention, the potential risk is to the offspring. Although results in Nonhuman primates (NHPs) are reassuring, some effects may not manifest for many years.”
Germline modification raises the issue of human dignity. Article 1 of Universal Declaration on the Human Genome and Human Rights says, “The human genome underlies the fundamental unity of all members of the human family, as well as the recognition of their intrinsic dignity and diversity.” “Heritage of humanity”: this is how the human genome is viewed by UNESCO, stressing upon the unique and priceless asset which must be preserved and passed on to posterity.” Human genome is the common binding thread that runs through the entire humanity. Any attempt to alter it will lead to eugenics (methods of improving humans) i.e., making designer babies directly affecting the inherent dignity and equality of all human beings.
Despite the ethical concerns, research in germline modification and gene editing is advancing steadily. “Gene editing in humans, including the CRISPR/Cas9 (Clustered Regularly Interspaced Short Palindromic Repeats/Cas9) technique, already forms part of the molecular tools available to researchers and has increased its use among the scientific community over recent years.”
Organ Donation or Egg donation?
Another contentious issue pertains to categorization of MRT. Is it to be treated as organ donation or egg donation? This determination is indispensable because of their different ethical and legal implications. By calling it mitochondrial replacement which in reality is much more than that-actually it is ‘enucleated’ egg donation- some scientists and regulators want to include it under the category of organ donation. Haimes & Taylor suggest an exact label would be “nuclear DNA hosting”. In this regard, Dimond & Stephens state, “Haimes & Taylor (2015) highlight how the donor had become an absent presence, where her role was minimised, distanced and rendered invisible as part of a political strategy. They suggest one layer to this is the terminology of ‘mitochondrial donor’, which implied that it was only mitochondria she provided, rather than the other cellular structures in the egg.”
Thus there seems to be a deliberate underplaying of the donor’s role in the procedure, notwithstanding her substantial contribution. On the contrary, her willing acceptance of pain and suffering brought on by the hormonal injections, their side effects, and egg retrieval need greater appreciation.
As with other assisted reproductive methods involving third party, such as surrogacy or egg/sperm donation, MRT also raises the issue as to whether the offspring possess the right to be told of their genetic contributors and lineage, thereby jeopardizing contributor privacy. In this respect the UK Regulations, 2015 do not allow identification of mitochondrial donors by citing following reasons:
“A key reason for encouraging clinics to disclose non-identifying donor information to patients and parents of donor-conceived people is to help parents share information about their child’s genetic origins, and to prepare them for potentially meeting their donor once they can receive donor identifying information at 18. These Regulations introduce a different system for mitochondrial donor-conceived children because it is recognised that a mitochondrial donor does not determine the characteristics of a child in the same way as with gamete donation and will not be the genetic parent of any child born. The Regulations specify that mitochondrial donors cannot be identified, reflecting the policy view that mitochondrial donation is more akin to organ donation than egg or sperm donation.”
Thus, the HFEA has skirted around the issue of classifying mitochondrial donation as either organ donation or gamete donation by creating a third category and describing it as “more akin to organ donation than egg or sperm donation.” This ambiguity in language does not dilute the fact that MRT leads to alteration of the cellular genome which, in case of a female child, is going to be transmitted to coming generations, thereby resulting in germline modification. MRT does seem to have all the characteristics of gamete (egg) donation and therefore deserves to be categorized as such.
Donor’s Influence on the Child’s Personality and Identity
That the mitochondria donor makes a significant contribution towards the birth of a healthy child despite providing less than 0.1% of the child’s genome is evident from the fact that mtDNA is crucial for the child’s physical and mental wellbeing. To that extent, the donor does influence the child’s growth and development, and actual shaping of its personality. Therefore she fully deserves medically as well as legally the title of a third parent. As such, the issue of her parental rights needs to be considered along the lines of established legal norms. A complex issue as to what extent the donor genes influence the personality of the offspring is debatable. Some claim that the genetic parents provide through their nuclear DNA more than 99.9% of total genes of the child, which contain the code for physical traits and characteristics responsible for the identity of an individual in which the mtDNA has no role to play.
However, to quote Baylis, “Identity is not in the genes but in the world in which we live and the stories we construct and are able to maintain.” Donor’s healthy mtDNA is going to influence the personality and the identity of the child who would have otherwise been born with a mitochondria linked genetic disease. According to Annelien Bredenoord, “…A person without amtDNA disease will have a different life experience, a different biography and perhaps also a different character”.
Mitochondrial Influence on Ageing and Longevity
Some studies indicate that ageing and longevity as well as athletic prowess of an individual might be influenced by mtDNA. “It is quite likely that dysfunction in mitochondrial metabolism can, to some extent, be a cause of ageing and, consequently, that mitochondrial determinants of ageing can in turn be detrimental for longevity.” There is constant interaction between mitochondria and nucleus of a cell. Consequently, certain changes are brought about in the nDNA by the mtDNA, which suggests that nDNA which shapes a person’s identity is itself influenced by mtDNA.
Therefore to say that mtDNA has no role in the identity of an individual will be oversimplification of facts. Mitochondria are energy providers for cellular functions because of which they create a much wider and deeper impact on the physiology and thereby on the identity of an individual despite contributing less than 0.1% of their total genes.
“Mitochondria play an important role in many bodily processes, and therefore the genetic contribution of the donor might be significant: there are complex interactions between nuclear DNA and mitochondrial DNA” “and organelles contained in the cytoplasm might introduce epigenetic alterations in nuclear DNA.”
According to Rettner (2013), “Epigenetics literally means above or on top of genetics. It refers to external modifications to DNA that turn genes on or off. These modifications do not change the DNA sequence, but instead, they affect how cells read genes.” The changes are reversible and can be transmitted when the cell divides.
Thus it is observed that mtDNA has a far deeper impact on the nDNA, and, a genetic mismatch between the two may likely produce consequences detrimental to the normal cellular function. However, an expert panel states that the risk of deleterious consequences of mito-nuclear reactions and harmful epigenetic effects is theoretical and there is no evidence of actual risk.
Harm to the Child and Posterity
The technology is at an experimental stage and uncharted waters where there is a big question mark about its safety and efficacy. The harmful effects of combining two different sources of DNA may not manifest in the short term. The root cause of the malady sought to be prevented viz. the faulty mtDNA is not completely eliminated and may ‘bounce back’. The HFEA found that the MRT techniques were ‘not unsafe’. The words seem to lack conviction and left many wondering about the safety. MRT may induce new mutations which may remain undetected for years. Potential genetic harm to future generations cannot be envisaged at this stage; however, social conflicts and inequality seem to cast a shadow.
Harm to the Egg Donor
The donor has to undergo a time consuming process of visits to the clinic for filling up the forms, interview, screening and learning to self-inject the medication. The hormone induced ovulation and the egg retrieval are painful. Aside from money paid she is hardly given any recognition for all the trouble she underwent, especially for her invaluable genetic contribution to the child. The hormones can cause cramps, haemorrhage, abdominal pain; mood swings etc. (Ovarian Hyperstimulation Syndrome) while more serious effects include damage to other organs, and cancer. The risk incurred by far exceeds the reward received, exposing her to exploitative and coercive tactics especially if she comes from lower economic strata.
Impact on the Society
Application of the technique other than for preventing genetic disorders transmitted through faulty mtDNA looms large. Already in Ukraine it has been used to treat infertility, a use not originally intended. If both the partners in a lesbian union are desirous of linking with their child genetically, they will easily be tempted to use this technique. The technique of PNT where “two embryos are destroyed to create a third” one may not be acceptable to many on ethical grounds as it violates the sanctity of human life.
The human genome is presumed to have originated from a single ancestor and has been preserved throughout the human evolution. This continuity will be disrupted by human interference with the genome. As the mtDNA is passed on only through females to future generations, it will permanently alter the maternal line; posing difficulty for those desirous of tracing their ancestry in order to gain genealogical information.
The possibility of genetic enhancement, i.e., “creating improved humans” is closely linked to germline modification. With the portent of Trans-humanism (Aiming to transcend beyond human bodily limitations), the danger of crossing the ethical boundaries and indulging in selection of genes (“designer babies”) in order to enhance performance is palpably close. Two other factors may further impact- both related to the economics of the technique. Because of its exorbitant cost, the procedure will be available only to select few, resulting in unequal access and impediment to distributive justice. Secondly, exploitation and/or coercion of economically weaker women-as was seen in commercial surrogacy-cannot be avoided owing to very large amounts of money paid to the mitochondrial donors.
Miscellaneous Considerations
Pro Nuclear Transfer (PNT) involves destruction of two embryos to produce a presumably healthy third one. In the UK where the procedure enjoys legal sanctity, this has led to commodification of human embryos as spares, and, through them essentially, of egg donors i.e., women as suppliers. Such reckless approach is recipe for untold harm, putting entire society in peril by unleashing market forces and providing fertile ground for business enterprises to flourish. Although Maternal Spindle Transfer (MST) does not involve destruction of embryos, a number of embryos are created from amongst which the one most likely to survive is selected while the rest are discarded. During the transfer, a “carryover” of 1% to 2% of faulty mitochondria can occur which may pose problems in future.
Mitochondria are the batteries or powerhouse of a cell, providing energy for its various functions. Hence they are most likely to affect cellular metabolism and ageing, leading to increased risk of cancer. Therefore, in the interest of science, and humanity at large, it is necessary to follow up over a long period not only the babies born via this procedure but their progeny as well. Such follow up over long term requires participants’ incentivisation as they become “lifelong guinea pigs”. The procedure is only preventive, and not curative. It does not repair or rectify the faulty mitochondria in the mother. Moreover, parents have no reason to suspect anything abnormal until the first child is born with the disease. As such, an effective preventive measure would comprise of surveillance, screening, and risk assessment. Although overall incidence is 1 in 200, severely affected cases are few in number viz.1 in 10000 to justify the use. The therapy is expensive and has many known-unknowns, making the risk-reward ratio unfavourable.
Ethical ‘Slippery slope’
A minor ethical breach starts a pattern which, over a period of time, leads to major transgression as each time the ethical boundary is stretched little further until the bottom is reached. Therefore, the only prevention from going down the slippery slope is not to take the first step in that direction. In this case, the argument is based on the assumption that the demarcating line between therapeutic and nontherapeutic application of germline modification can and will be transgressed in due course of time and the much dreaded application of the technique for genetic enhancement will become a reality. The critics argue that the argument is built on plausibility, not certainty; and regulations put in place will prevent such an occurrence. The supporters of the idea question whether regulatory restraints will be able to stop the descent down this slope. The restraints can at best slow down the speed of descent but will not arrest it.
Regulations are not carved in stone. Their existence is as impermanent as the cultural notions and beliefs they are based on. Once germline editing is available, it might beextended to “improving” humans; in turncreating a market for such services, attracting providers, users, investments and pressure groups.
The National Academies of Sciences, Engineering, and Medicine, Washington, DC report on human genome editing (2017) states, “Overall, slippery slope arguments do not depend on universal condemnation of the initial, most compelling applications of heritable genome editing. But while many think that regulation could establish effective speed bumps, proponents of slippery slope arguments raise the question of whether and how society can develop regulations that are sufficiently robust to quell the fear of a progressive move toward less compelling and more controversial applications. Indeed, they would say that regulations would do little to stop the progression down a slippery slope because regulations are based on cultural views, and it is precisely the underlying change in cultural views that is the slippery slope.” The change in views can cause “moral disengagement” which leads to overcoming of the inhibitory controls that guard against unethical behaviour, and encourages unethicality incrementally. “Gradual changes can increase moral disengagement and unethical behavior over time. However, the effectiveness of a prevention focus in reducing unethical behavior suggests that there may be other important individual and contextual factors that influence one’s susceptibility to this phenomenon. Small instances of unethical behavior may begin to snowball into larger violations.”
LEGAL DIMENSIONS
Legalisation of Research and its Clinical Application
So far only the UK has legalised the treatment via MRT for which both the procedures- MST and PNT have been granted approval. The US Congress has banned since 2015 clinical trials of the technique “in which a human embryo is intentionally created or modified to include a heritable genetic modification.” Australia permits limited research in MRT on embryos not more than 14 days old, but at the same time bans its application in clinics. The only country that does not expressly ban human germline modification is Thailand, while Israel and Italy allow it conditionally. In Greece as well as in Ukraine the procedure is used to treat infertility.
The Human Fertilisation and Embryology Authority of UK grants permission to use MRT only for preventing transmission of genetic disease via mutant mtDNA and not for infertility. Each case is individually decided on merit. So far permission was granted in 14 cases but no birth of a baby conceived via MRT has been reported in the UK. In the US there has been a concerted effort to lift the ban. Some scientists, interest groups want the ban removed to enable female carriers of mutant mtDNA to have normal, genetically linked babies. However, the parental rights to have a genetically linked baby through this technique must be balanced against long term interests of the society wherein, according to some, the scales tilt towards the latter. Some others call the procedure a kind of cloning where nuclear genetic material is transferred to an enucleated egg. In that case the ban cannot be lifted.
However, the National Academies of Sciences, Engineering and Medicine in its report titled Mitochondrial Replacement Techniques: Ethical, Social, and Policy Considerations (2016) examines in depth the issue of MRT and finds that “it is ethically permissible to conduct clinical investigations of MRT” (p.108), albeit with restrictions on its use and subject to certain conditions. It also recommends that the technique be used initially only for male embryos to avoid “creating heritable genetic modification.”
The International Society for Stem Cell Research professional guidelines (2016) state, “Mitochondrial replacement therapy employs distinct methods and does not entail direct modification to the nuclear genome. Preclinical research into the safety and efficacy of mitochondrial replacement strategies is now underway and should continue under appropriate regulatory oversight.”
The Oviedo Convention: Protection of Human Rights in Biomedicine
This European Convention has been signed by 35 countries but ratified by 29 of them on whom it is binding by implementation through domestic laws. Based on the European Convention on Human Rights in the field of Biology and Medicine, it aims to protect human dignity and individuality. Art. 13 of this instrument lays down that “an intervention seeking to modify the human genome may only be undertaken for preventive, diagnostic or therapeutic purposes, and only if its aim is not to introduce any modification in the genome of any descendants.” (Emphasis added)
A group of experts across the globe in 2015, while favouring conditional acceptance of human genome editing, stated, “We recommend that a detailed but flexible roadmap is produced to guide the development of standards for safety and efficacy”. The French National Academy, while foreseeing a possibility of heritable genome editing being permitted in future, states, “This research, including that on germline cells and human embryos, should be carried out provided that it is scientifically and medically justified.”
These bodies seem to agree that application of heritable genome editing must not be allowed until all the questions as to its safety and efficacy are answered satisfactorily; however, with science making rapid advances in this area, none of them favours a permanent ban.
Informed Consent
Informed consent is defined by The American Medical Association as follows, “The process of informed consent occurs when communication between a patient and physician results in the patient’s authorization or agreement to undergo a specific medical intervention.” The information to be presented by the physician must include nature and aim of the treatment, possible risks, benefits, and treatment alternatives. However informed consent in an experimental therapy is complicated. The amount of information to be disclosed is a vexing issue here. An information overload may dissuade a likely participant whereas suboptimal disclosure breaches the Nuremberg Code and Declaration of Helsinki, which require fully informed consent.
Regarding the documentation of informed consent obtained by Dr. Zhang and his team in carrying out the MRT, five researchers on the editorial board of the journal Reproductive Biomedicine Online have criticized the consent form in observing that, “Consent form discussed MRT only in a superficial manner. Risks specific to mitochondrial replacement were not included. The authors explain that the patient received extensive counselling over the course of several years, but the final consent form does not record this. Although the egg donor reportedly signed a standard egg donor consent form, a copy of this form received by RBMO shows that the use of the donated eggs specifically for spindle transfer (for MRT) is not mentioned”. These observations serve a valuable insight into the procedural lapses that may occur in future, and indicate the way forward in ensuring strict compliance with regulations with respect to informed consent.
Organ Donation or Gamete Donation?
If mitochondrial donation is to be treated as organ donation then the donor relinquishes all the rights and responsibilities towards the donated organ and has no proprietary interest in the donated material on its “full integration” into the recipient’s body. But the ambiguity as to the point of time at which this integration occurs has led the courts to pronounce that the donor must clearly instruct, in advance, the medical professional as to the use of the donated organ, failing which it will be treated, on removal, as abandoned material. This proposition holds true even after any value addition to the said material is made with the use of advanced bio-techniques. In Moore v. Regents of the University of California, the California Supreme Court ruled that when material is extracted from a patient’s body, he loses all proprietary interest in that material.
In mitochondrial donation the donor egg is enucleated i.e., it is subjected to a scientific process which converts it into a different entity. Moore appears to laydown that after the eggs are removed from the donor, she has no property rights in them, unless previously agreed to the contrary. Although MRT is classified as a species of Assisted Reproductive Technologies (ART) which involve gamete (sperm, egg or embryo) donation, the procedure of MRT bears resemblance to organ donation and hence calls for adoption of legal treatment on the lines similar to those of organ donation.
Donor Anonymity
The UK law (Regulation 11 of Human Fertilisation and Embryology (Mitochondrial Donation) Regulations 2015) mandates anonymity of mitochondrial donor in contrast to the open identity status of gamete donors in other forms of ART. This differential treatment of the two types of donors arises from the earlier mentioned position that donation of mitochondria is rather donation of an organ than donation of a gamete. This view also prepares the ground for the argument against vesting parental rights in the mitochondrial donor in spite of her genetic contribution to the baby. The UK law frees the gamete donors as well as neither mitochondrial donors from parental obligations towards donor conceived children who can claim neither financial support nor inheritance from the donors. There are significant commonalities such as passing on of personal characteristics and a sui generis genetic relatedness, and not insignificant variances between gamete donation and mitochondrial donation. In due course of time, as further research sheds more light on the issue of relative weightage to be given to the commonalities and the variances, the question of anonymity of mitochondrial donors will need to be revisited. Brandt offers justification for maintaining the anonymity by suggesting that formal obligatory disclosure may deter couples in need of MRT from using it and they may risk having a child with mitochondrial disorder.
However, this policy is viewed as understating the mitochondrial donor’s role and overestimating the putative parental discomfort with disclosure of donor identity. Appleby finds that considering the large number of conceptions taking place in UK through gamete donation despite the disclosure of gamete donor identity, couples in need of MRT will not be dissuaded from availing it only because of the open identity status of mitochondrial donors, and recommends that the veil of anonymity be lifted from mitochondrial donors just like gamete donors and they be declared open identity donors.
It can be easily argued that notwithstanding the small amount of her genetic contribution, the mitochondrial donor plays a significant role in bringing into this world a healthy child, which, but for her, would not have been possible, and the child has every right to know its genetic contributor. Moreover, in ART, truth and transparency must be given primacy over deception and secrecy. Therefore, mitochondrial donation, like gamete donation, must be given open identity status.
Assigning Parentage
In the UK, the law does not consider a mitochondrial donor as a parent of the donor conceived child. This legal position was crafted on the recommendation of various bodies. The HFEA explanatory document (2015) states, “A mitochondrial donordoes not determine the characteristics of a child in the same way as with gamete donation and will not be the genetic parent of any child born.”
An independent institution working closely with the UK government, the Nuffield Council on Bioethics (2012) stated, “it is the view of the Working Group that mitochondrial donation does not indicate, either biologically or legally, any notion of the child having either a third parent, or second mother”. Concluding that the “mitochondrial donor was not a ‘second mother’ and the term ‘three parent families’ was ‘completely inappropriate’,” the Department of Health of UK, a regulator of biomedical matters, in its report states, “In using these techniques, the resulting child will have nuclear DNA (99.9 per cent) from their father and mother and healthy mitochondrial DNA (0.1 per cent) from a female donor. Genetically, the child will, indeed, have DNA from three individuals but all available scientific evidence indicates that the genes contributing to personal characteristics and traits come solely from the nuclear DNA, which will only come from the proposed child’s mother and father. The donated mitochondrial DNA will not affect those characteristics.”
It is observed from the above that all the institutions are unanimous in accepting the genetic relatedness between the donor of mitochondria and the ensuing baby, and rejecting parental status to the donor. Such a policy formulation points to the desire to perform a balancing act between deferring to the cultural significance of genetic relatedness and nullifying the possibility of future legal tangle that may arise if the mitochondrial donor is recognised as having parental rights. Thus, by labelling the mitochondrial donation as organ donation, maintaining donor anonymity, and rejecting parental status to the mitochondrial donor the UK is well positioned to handle the legal dimensions of MRT. This legislative provision is akin to Bright line rule applied in the US courts “setting a basic standard that clarifies an ambiguity and establishes a simple response. Often a bright line is established when the need for a simple decision outweighs the need to weigh both sides of a particular issue.” “A bright-line rule is easy to administer and produces certain, though, arguably, not always equitable results.” From these definitions it is seen that application of Bright line rule without a balancing test (Fine line rule), while gaining on simplicity but losing on equitability, does not always result in a fair outcome.
Although MRT is banned in the US, there is a strong push for it to be legalised. MRT is classified as a form of ART, but because of a different scientific technique involved in MRT, the laws governing ART must not be applied to MRT. According to Green, courts assign parentage in ART cases by applying any of the following bases- statutes, genetic relatedness, best interest of the child, and intention of the intended parents. The intention test is the most frequently used approach which was laid down in Johnson v. Calvert where the Court, in dealing with the dispute between the gestational surrogate and the genetic mother, stated, “she who intended to procreate the child—that is, she who intended to bring about the birth of a child that she intended to raise as her own—is the natural mother under California law.” The intent test is to be used as a tie breaker between competing admissible claims.
But in K.M. v E.G. Court disregarded the intention test in a dispute between a lesbian couple where K.M. donated the egg and E.G. carried the pregnancy after in vitro fertilisation, there being a prior agreement that the sole parent would be E.G. after K.M. waived her parental rights. Stating that parentage determination by the court is not affected by the waiver, the court granted parental rights to both while finding that the two claims were not in mutual exclusion but in mutual collaboration as “K.M. does not claim to be the twins’ mother instead of E.G., but in addition to E.G.,” and ruled that the “Johnson intent test does not apply when there is no ‘tie’ to break.”
The case law clearly indicates that courts do not ascribe much significance to preconception waiver of parental rights, making it unenforceable; and that there is considerable grey area in the laws governing ART and in particular, egg donation leading to unpredictability of the case outcome. The term donor has become all encompassing, taking in its fold a variety of techniques used in ART, turning it into a ‘one size fits all’ concept leading to ambiguity. Hence it is absolutely necessary to clarify it. Black has suggested a distinction between a donor and a lender by proposing: “Clarification of the term donor is urgently needed in order to differentiate between the two categories of egg/sperm providers for ART, namely, true donors and what I would call intentional lenders of procreative genetic material. “A true donor is someone who contributes his or her gametes to someone else with no intention of parenting the resulting child; whereas an intentional lender of procreative genetic material is someone who contributes his or her gametes for the purpose of having a child whom he or she intends to parent. This differentiation coupled with the fact that in MRT the mtdonor’s egg undergoes substantial scientific alteration (the enucleated donor egg is fitted with a nucleus from the intended mother’s egg) turns the mtdonor into a true donor in every such instance. Therefore, a pragmatic approach to prevention of likely parentage disputes in MRT would be to lay down the law that a mitochondrial donor will not have parental rights and obligations to the resulting offspring. Setting an objective standard in this way will considerably reduce the chances of disputes and ensure speedy resolution of disputes if any.
Child Support
Although the intending parents make a preconception agreement with the egg donor waiving her parental rights and releasing her from financial obligations towards the support of resulting child, such contractual terms may not be enforceable. The leading case on this issue, Kesler vs. Weniger though concerning a sperm donor’s liability towards child support, seems to be equally applicable to an egg donor or a mitochondrial donor. Weniger contended that before conception he was released from financial obligations and only due to this term of agreement he donated the sperm. The court found that such agreements are non-binding since: “It matters not when an agreement to forego support occurred; the right to support is a right of the child, not the mother or father.” “It cannot be bargained away before conception any more than it can be bargained away after birth, nor can it be extinguished by principles of estoppel.”
Similarly, in Ferguson v. McKiernan the court, citing Kesler, ordered the sperm donor to pay child support even though he was released from support obligations by the intending mother prior to conception. This order was set aside in appeal on the grounds that his equal protection rights under the Constitution were violated in that sperm donors in a physician assisted in vitro fertilisation were not liable for child support while those donating without such assistance were held liable. However, in the absence of similar case law, it is doubtful if such protection from child support liability is available to egg donors. Moreover, The Uniform Parentage Act, 1973 protects the sperm donors but not the egg donors from such liability; although the 2000 amendment to the Act attempts to include egg donors as well, it is subject to judicial interpretation. Therefore, in the light of the above rulings and the statute, it seems that an egg donor runs the risk of incurring financial liability for the donor conceived child notwithstanding the fact that there is a preconception agreement absolving the egg donor from child support.
After taking into consideration all the above legal facets of mitochondrial donation, it seems prudent to make a law stating unequivocally that a mitochondrial donor will not be granted parenthood, intended or otherwise, of the baby resulting through such donation and such donor will be free from any pecuniary liability towards the child so conceived. Many a mitochondrial donor, whether altruistic or not, is likely to willingly undergo the pain and discomfort associated with egg retrieval procedure in order to donate the mitochondria if she is assured of complete freedom from any post facto legal hassle in connection with the donation.
As to the donor anonymity and privacy, the existing UK law (Section 11 of HFEA Regulations, 2015) permitting disclosure of non-identifying information to the child at 18 years of age appears to be quite reasonable and adequate at present. However, as the procedure becomes safer, popular and widely used, the law must permit, just as in case of a gamete donor, disclosure of mitochondrial donor identity to the child at 18. The following extract from UNESCO Report which emphasises on the ethical aspect of both scientific research and law making “while respecting the human rights to freedom and safety” sheds much light on the interconnectedness between responsible ethical research, its application, and equitable justice.
“We are human because of the interplay of many biological, historical, and cultural determinants, which preserve the feeling of our fundamental unity and nourish the richness of our diversity. The international community, States and governments, scientist, actors of civil society and individuals are called upon to consider the human genome as one of the premises of freedom itself and not simply raw material to manipulate at leisure. At the same time, considering that’s scientific advancements in this field are likely to offer unprecedented tools against diseases, it is crucial to acknowledge that these opportunities should never become the privilege of the few. What is heritage of humanity entails sharing both responsibilities and benefits.”
Conclusion
Germline therapy is here to stay. Although its application needs to be strictly regulated to prevent humanity from sliding down the ethical “slippery slope”, the fundamental research in gene editing need not be banned. On the contrary, fundamental research must be distinguished from its application, and allowed to be proceeded with. Responsible ethical scientific research must not be thwarted in its relentless pursuit of progress and advancement of knowledge. To that extent, the U.K. has shown the way for others by putting a stringent regulatory framework in place. Most of the ethical problems will get resolved as babies born via MRT are followed up for number of years, and the data accumulate. As the procedure gets more and more refined and sophisticated as a result of evolving technology, it will become safer for the stake holders viz. the mother, the egg donor, the baby and the society at large. However, the question is not whether we can do it, but whether we should do it. It will take time to find the answer. Till then, caution and circumspection should be the operative words.
The legal issues need to be addressed with utmost priority. Parental rights and obligations and their waiver, birth certificate of the child, financial liability of the third parent towards child support, donor privacy, should the identity of the third parent be revealed to the child?, type of contract i.e., tissue/organ donation or gamete (sperm/egg) donation, informed consent, etc. are some of the key issues for which law must find answers. Since the legislative process has to take its own course, the judiciary will have to step into the role of law maker to frame the rules in order to settle disputes arising from any of the above issues.
Even though it may be quite a few years before the technology becomes legally available and affordable to the public, a multi-disciplinary forum comprising of academicians from diverse fields like genetics, bioengineering, law, bioethics & philosophy, as well as government agencies must be constituted in earnest to initiate a meaningful discussion about its profound and lasting impact on humanity in general and posterity in particular. Unbeknown, future barrels down on us relentlessly.
References
- Académie Nationale De Médecine (2016). Genetic editing of human germline cells and embryos. Retrieved from http://www.academie-medecine.fr/.
- Alikani, M., Fauser, B.C.J., García-Valesco, J.A., Simpson, J.L., & Johnson, M.H., (2017). First birth following spindle transfer for mitochondrial replacement therapy: Hope and trepidation. Reproductive BioMedicine Online, 34(4), 333-336.
- Amato, P., Tachibana, M., Sparman, M., & Mitalipov, S. (2014). Three-Parent IVF: Gene replacement for the prevention of inherited mitochondrial diseases. Fertility and Sterility, 101(1), 31-35.
- American Medical Association. “Informed consent”. Retrieved from https://www.ama-assn.org/delivering-care/ethics/informed-consent.
- Appleby, J.B. (2018). Should mitochondrial donation be anonymous? Journal of Medicine and Philosophy, 43(2), 261-280.
- Baylis F. (2003). Black as me: Narrative identity. Developing World Bioethics, 3(2), 142-150
- Baylis, F. (2013). The ethics of creating children with three genetic parents. Reproductive Biomedicine Online, 26(6), 531-534.
- Black, L.W. (2014). The birth of a parent: Defining parentage for lenders of genetic material. Nebraska Law Review, 92(4), 799-842.
- Brandt, R. (2016). Mitochondrial donation and ‘the right to know’. Journal of Medical Ethics, 42(10), 678-684.
- Bredenoord, A., Dondorp, W., Pennings, G., & De Wert, G. (2011). Ethics of modifying the mitochondrial genome. Journal of Medical Ethics, 37(2), 97-100.
- Caldwell, S. (2015). Three-parent baby law makes human life disposable. Catholic Herald. Retrieved from https://catholicherald.co.uk/three-parent-baby-law-makes-human-life-disposable-says-bishop/.
- Coghlan, A. (2017). Questions raised over 3-parent baby procedure last year. Retrieved from https://www.newscientist.com/article/2126512-.
- Coghlan, A. (2017). Questions raised over 3-parent baby procedure last year. Retrieved from https://www.newscientist.com/article/2126512-.
- Convention for the protection of human rights and dignity of the human being with regard to the application of biology and medicine: Convention on human rights and biomedicine. Retrieved from https://rm.coe.int/168007cf98.
- Darnovsky, M. (2013). A slippery slope to human germline modification. Nature, 499(7457), 127.
- De Lecuona, I., Casado, M., Marfany, G., Baroni, M.L., & Escarrabill, M. (2017). Gene editing in humans: Towards a global and inclusive debate for responsible research. Yale Journal of Biology and Medicine, 90(4), 673-681.
- Public Health Directorate/Health Science and Bioethics Division/10250 (2014). Draft regulations to permit the use of new treatment techniques to prevent the transmission of a serious mitochondrial disease from mother to child. Retrieved from https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/332881/Consultation_response.pdf.
- Dimond, R. (2015). Social and ethical issues in mitochondrial donation. British Medical Bulletin, 115, 173-182.
- Dimond, R., & Stephens, N. (2017). Three persons, three genetic contributors, three parents: Mitochondrial donation, genetic parenting and the immutable grammar of the ‘three x x’. Health, 22(3), 240-258.
- Fischbach, R.L., Benston, S., & Loike, J.D. (2014). Creating a three-parent child: An educational paradigm for the responsible conduct of research. Journal of Microbiology & Biology Education, 15(2), 186-190.
- Green, D.R. (2018). Assessing parental rights for children with genetic material from three parents. Minnesota Journal of Law, Science & Technology, 19(1), 251-276.
- Greenfield, A. (2016). Scientific review of the safety and efficacy of methods to avoid mitochondrial disease through assisted conception: 2016 update. Retrieved from http://www.hfea.gov.uk/docs/Fourth_scientific_review_mitochon dria_2016.PDF.
- Guidelines for Stem Cell Research and Clinical Translation. Retrieved from https://www.isscr.org/docs/default-source/all-isscr-guidelines/guidelines-2016/isscr-guidelines-for-stem-cell-research-and-clinical translationd67119731dff6ddbb37cff0000940c19.pdf.
- Haimes, E., & Taylor, K. (2015). Rendered invisible? The absent presence of egg providers in UK debates on the acceptability of research and therapy for mitochondrial disease. Monash Bioethics Review, 33(4), 360-378.
- Hamzelou, J. (2016). Everything you wanted to know about ‘three parent’ babies. Retrieved from https://www.newscientist.com/article/2107451.
- Complete list of the council of Europe's treaties. Retrieved from https://www.coe.int/en/web/conventions/full-list/-/conventions/treaty/164/signatures.
- Regulating mitochondrial donation (2015). Retrieved from https://www.hfea.gov.uk/media/1537/regulating-mitochondrial-donation.pdf.
- Human Fertilisation and Embryology Act 2008 (HFEA 2008):2015 Regulations.
- Knoppers, B.M., Nguyen, M.T., Noohi F., & Leiderman, E. (2018). Human genome editing ethical and policy considerations – policy brief. Retrieved from http://bit.ly/2hi1pAR.
- Zhang, J., Liu, H., Luo, S., Lu, Z., Liu, Z.,… & Huang, T. (2017). Live birth derived from oocyte spindle transfer to prevent mitochondrial disease. Reproductive Biomedicine Online, 34(4), 361-368.
- Loike, J.D., & Reame, N. (2016). Opinion: ethical considerations of “Three-Parent” babies. Retrieved from https://www.the-scientist.com/critic-at-large/opinion-ethical-considerations-of-three-parent-babies-32320.
- Mullin, E. (2019). Patient advocates and scientists launch push to lift ban on ‘three-parent IVF’. Retrieved from https://www.statnews.com/2019/04/16/mitochondrial-replacement-three-parent-ivf-ban/.
- National Academies of Sciences, Engineering and Medicine (2016). Mitochondrial Replacement Techniques: Ethical, Social, and Policy Considerations. Washington, DC: The National Academies Press. Retrieved from https://doi.org/10.17226/21871.
- National Academies of Sciences, Engineering and Medicine (2017). Human Genome Editing: Science, Ethics, and Governance. Washington, DC: The National Academies Press. https://doi.org/10.17226/24623.
- New genetic technologies in human beings and Human rights. Retrieved from http://www.alliancevita.org/
- Nuffield Council on Bioethics (2012). Novel techniques for the prevention of mitochondrial DNA disease: An ethical review. Retrieved from http://www.nuffieldbioethics.org/sites/default /files/Novel techniques for the prevention of mitochondrial_ DNA_diseases_compressed.pdf/.
- Prentice, D.A. (2015). Mitochondrial replacement therapy and 3 parent embryos. Retrieved from https://lozierinstitute.org/testimony-of-dr-david-prentice-ph-d-before-institute-of-medicine-on-mitochondrial-replacement-therapy-and-3-parent-embryos/.
- Pritchard, S. (2018). Australian Senate endorses mitochondrial donation – BioNews. Retrieved from https://www.bionew s.org.uk/page_136808.
- International Bioethics Committee (2015). Report of the IBC on updating its reflection on the human genome and human rights. Retrieved from https://pubmed.ncbi.nlm.nih.gov/27311162/.
- International Bioethics Committee (2015). Report of the International Bioethics Committee (IBC) on Updating Its Reflection on the Human Genome and Human Rights. Retrieved from https://pubmed.ncbi.nlm.nih.gov/27311162.
- Rettner, R. (2013). Epigenetics: Definition & Examples. Retrieved from https://www.livescience.com/37703-epigenetics.html.
- Sandy, O. (2018). Singapore could become the second country to legalize mitochondrial replacement therapy. Retrieved from https://www.sciencemag.org/news/2018/06/singapore-could-become-second-country-legalize-mitochondrial-replacement-therapy.
- Santoro, A., Salvioli, S., Raule, N., Capri, M., Sevini, F.,… & Franceschi, C. (2006). Mitochondrial DNA involvement in human longevity. Biochimica et Biophysica Acta, 1757(9-10), 1388-1399.
- Sharma, H., Singh, D., Mahant, A., Sohal, S.K., Kesavan, A.K., & Samiksha. (2020) Development of mitochondrial replacement therapy: A review. Heliyon, 6(9).
- Statement on genome editing technologies and human germline genetic modification. Retrieved from http://www.hinxtongroup.org/hinxton2015_statement.pdf.
- Terman, S. (2008). Marketing motherhood: Rights and responsibilities of egg donors in assisted reproductive technology agreements. North-western Journal of Law and Social Policy, 3(1), 167-184.
- The human fertilisation and embryology (Mitochondrial Donation) regulations (2015). Regulation 15 modifying Section31ZE of HFEA, 2008. Retrieved from http:// www.legislation.gov.uk/uksi/2015/572/.
- Third scientific review of the safety and efficacy of methods to avoid mitochondrial disease through assisted conception, Human Fertilisation and Embryology Authority, June 2014, as cited in Saey, T.H. (2016). Three-parent-babies-explained. Retrieved from https://www.sciencenews.org/article/three-parent-babies-explained.
- Trials of War Criminals before the Nuremberg Military Tribunals under Control Council Law No. 10 (Volume 2). Retrieved from https://collections.nlm.nih.gov/catalog/nlm:nlmuid-01130400RX2-mvpart.
- UNESCO: Universal Declaration on the Human Genome and Human Rights, adopted by acclamation at UNESCO's 29th General Conference (1997), endorsed by the United Nations General Assembly in 1998. Retrieved from http://www.unesco.org/new/en/social-and-human-sciences/themes/bioethics/humangenome-and-human-rights/.
- Welsh, D.T., Ordóñez, D.L., Snyder, D.G., & Christian, M.S. (2015). The slippery slope: How small ethical transgressions pave the way for larger future transgressions. Journal of Applied Psychology, 100(1), 114-127.
- World Medical Association (2013). Declaration of Helsinki ethical principles for medical research involving human subjects. Retrieved from https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/.
- Word of the day. Retrieved from https://www.dictionary.com/.
- Zhang, J., Liu, H., Lu, Z., Luo, S., Chávez-Badiola, A.,… Huang, T. (2017). Live birth derived from oocyte spindle transfer to prevent mitochondrial disease. Reproductive Biomedicine Online, 34(4), 361-368.
- Kesler vs. Weniger, 744 A.2d 794 (Pa. Super. Ct. 2000).
- Moore vs. Regents of University of California, 793 P.2d 479 (Cal. 1990).
- Johnson vs. Calvert, 851 P.2d 776 (Cal. 1993).
- Ferguson vs. McKiernan, 855 A.2d 121 (Pa. Super. Ct. 2004).
- Ferguson vs. Mckiernan, 868 A.2d 378 (Pa. 2005).
- K.M. vs. E.G. 117 P.3 d 673 (Cal. 2005)