Original Articles: 2017 Vol: 16 Issue: 1
Pricing and Assessment of Competitiveness of Innovative Medical Devices in the Context of Commercialization Strategy
Tatiana Jurievna Kudryavtseva, Peter the Great Saint-Petersburg Polytechnic University
Elena Andreevna Ivanova, Peter the Great Saint-Petersburg Polytechnic University
Evgeniia Alexandrovna Kozlova, Peter the Great Saint-Petersburg Polytechnic University
Angi Erastievich Skhvediani, Peter the Great Saint-Petersburg Polytechnic University
Keywords
price, competitiveness, import substitution, commercialization, devices for medical use.
Introduction
At the moment, the Russian Federation is implementing the strategy of innovative development of the economy and import substitution in healthcare. In this context, Russian researchers develop products and technology, including those for medical use, both on the basis of foreign analogues and unique ones, with the degree of completion of developments varying within a wide range – from an idea to a prototype.
Healthcare is the most important area of life support; the results of its functioning shape the quality of life. In 2014, total consumption of medical products in the Russian Federation amounted to 199.6 bln rub. of which 78% fell on the consumption in the public sector.
At the same time, only 16.6% (33.2 bln rub.) fell on the medical products of domestic production, while the remaining 83.4% fell on the imported products (STC MEDITEX, 2015). As such, the predominance of imports in consumption and supply of the medical products on the Russian market leads to a dependence of prices for vital devices on the international economic and political situation. In our opinion, this area should remain stable and to a minimal degree depend on changes in the economic and political situation in the world. Achievement of such a state requires targeted shaping of national security in healthcare and ensuring its import independence.
According to the Order of the Ministry of Industry and Trade of the Russian Federation dated January 31, 2013 No. 118 on the approval of the development strategy for the medical industry of the Russian Federation for the period through to 2020, 10% annual growth in the volumes of the medical products market are expected since 2015. As part of the scenario of organic growth, the volume of the market for medical devices will reach 340 bln rub. by 2020. At the same time, the share of domestic medical products in consumption should increase to 40%, the share of exported domestic products should increase to 16.3%, the share of companies implementing technological innovations should increase to 50%, and labor productivity in the medical industry should double the level of 2012.
As such, the efficient solution of the task of import substitution in healthcare requires to ensure the promotion of promising developments from research laboratories into practice and their implementation with minimal cost, as well as to form the resource base for continued research and development of the “research – production – consumption” chain in order to further improve the equipment of healthcare.
However, the "research for research" model is currently being implemented in the Russian economy, when they remain non-demanded on the market after the execution of grants. According to data from NBK Group, the share of commercialized patents has been declining in Russia from 2004 to 2012. In 2012, the figure reached the value of 0.14%, which is 2/3 less than in 1992 (NBK Group, 2013). According to data from HSE, 2,965 internal agreements on trading licenses and alienation of patents were registered in 2014, of which 14.8% fell on the agreements in the area of medical equipment (Gorodnikova, N.V, Gokhberg, L.M., Ditkovskiy, K.A. et al., 2016). According to the federal target program "Development of the pharmaceutical and medical industry of the Russian Federation for the period of 2020 and beyond", the figure "volume of production of modern domestic medical products at the expense of commercialization of the developed technology" reached the target value of $0.8 bln rub. in 2014, was fulfilled by 44.8% and amounted to 1.3 bln rub in 2015. We must note that this figure should rise 41.3 times by 2020 as compared to 2015 and reach 53.7 bln rub. As such, the real growth rate is much lower in 2015 than the target. On the other hand, the academics note that Russian universities should both develop scientific-innovative activity of universities (Rodionov, D. G., Rudskaia, I. A., & Kushneva, O. A., 2014) and address issues of promotion and incorporation into the international academic system (Rodionov, D. G., Fersman, N. G., & Kushneva, O. A., 2016) (Rodionov, D. G., Rudskaia, I. A., & Alexandrovna, K. O., 2014). Consequently, in the absence of changes in the current model of research, the gap between target and actual figures will increase, and research of the domestic scientists will remain non-demanded on the market.
Based on the above, the achievement of the goals presented in the strategy of development of the medical industry through to 2020 requires organizational and industrial mechanism of consolidation of business, state and academic community in the development of the medical industry. This goal can be realized through the development of the complex of methodical support of the implementation and commercialization of domestic innovative devices and technology of medical use, meeting the interests of representatives of the business, government agencies and researchers. This will contribute to the achievement of the strategic goals at the expense of improving the efficiency of selection of promising innovative developments, optimization of the process of their implementation, distribution focused on the complex socio-economic result, and commercialization of innovations.
Issues of evolution of models and processes of development of innovative medical devices are widely discussed in the scientific literature by Wood B. (1996), Rudelius W. (1997), Alexander K., & Clarkson P. J. (2002), Imelli P. & Kobe C. (2008), Mehta S. S. (2008), Shah S. G. S. & Robinson I. (2008), Panescu D. (2008), Rochford L. & Medina L. A., Kremer G. E. O. & Wysk, R. A. (2013), Santos I. C. (2013), and Philips Plastic Corporation (2013). It was found based on the analysis of papers of these authors that the assessment of the potential of commercialization of innovative devices for medical use must be accompanied by the processes of testing the hypotheses about the demand for design, configuration and consumer-oriented characteristics by the main consumers and users of the device. In particular:
1. At the preparatory stage, when the idea is generated, the overall concept of the device is determined and a project plan created, it is required to: conduct a study of regulatory requirements to the characteristics of the device, compare its hypothetical characteristics with the characteristics of the analogues that exist today, predict their development in the future, define a niche the device will take in the future using the price-quality parameters, which consumer-oriented characteristics will be most in demand on the market;
2. In the process of the device development, it is required to control the correspondence of actual device characteristics to those defined at the preparatory stage, test pilot samples of the device and convert the users and consumers feedback into measures to improve the quality of the device and to bring its characteristics in correspondence to the ones required by the market;
3. At the stage of the creation of the device and its launch on the market, it is required not just to conduct its clinical trials in order to determine its compliance with all necessary regulatory requirements and the final formalization of its characteristics and the effect on a patient, but also to define production specifications, plan and methods of production, supporting processes of marketing, packaging, logistics, etc.
As such, each stage of the device development sets various goals for the team of researchers, the achievement of which is required for a successful launch of the device on the market. As a consequence, the organizational and methodical complex must contain a set of tools adapted to the specific goals and objectives for each stage. In this case, the process of the device creation will be synchronized with both the evolution of market requirements to the characteristics of the device and to the changes in the regulatory and institutional nature, which will increase the chances of its successful commercialization.
The reviewed methodical bases of commercialization of innovative medical devices will be tested on the example of a mobile device for express diagnosis of myocardial infarction biomarkers. The prototype of this device was designed by the research team of the Center of Microtechnology and Diagnosis of the Saint-Petersburg State Electrotechnical University "LETI" named after V.I. Ulyanov-Lenin (hereinafter SPbSETU) within the grant "Development of physical and chemical foundations and creation of the sample of the analytical micronanobiosystem for preclinical express identification of myocardial infarction biomarkers" from the Ministry of Education under the federal target program "Scientific and academic human resources of innovative Russia" (Agreement No. 14.V 37.21. 0793 dated September 03, 2012) (Pat. 2280247 of the Russian Federation, MPK7 G01N21/64, G01N21/03) (Luchinin V.V., Zimina T.M., 2013).
As part of this study, the consumer characteristics of the domestic express analyzer of the critical states developed in SPbSETU "LETI" were assessed, the optimal price was defined taking into account the level of its competitiveness, and the optimal device characteristics from the perspective of the consumer were defined.
Methods
The key goal of the study was to quantitatively assess the competitiveness of the domestic express analyzer of critical conditions, as well as to determine the optimal price for the device, taking into account the calculated level of competitiveness. The "indifference price" method was used to do so, which allows to define the level of price for the product, at which a unit of useful effect of one product and the product of competitors will have the same price. In other words, the price is defined, at which the buyer will have no difference which product to purchase: the product in question or the competing one (Lifits, I.M., 2014).
Assessment of competitiveness and determination of the indifference price is made in several stages:
1) Market analysis, identification of key competitors.
2) Identification of the most important technical and consumer characteristics of the device.
3) Determination of the level of importance of characteristics for the end user as a weight index (index of importance), 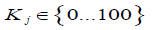 expressed as a percentage. The weight index can be calculated as the arithmetical average assigned to the selected technical characteristics using the method of expert assesment:
expressed as a percentage. The weight index can be calculated as the arithmetical average assigned to the selected technical characteristics using the method of expert assesment: 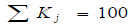
4) Determination of the product characteristics, optimal for the consumer, in accordance with the wishes and preferences of consumers, i.e. identification of the characteristics of the "ideal" product.
5) Assessment of the degree of correspondence of each parameter of the assessed product to characteristics of the "ideal" product as percentage: 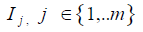
6) Calculation of the average parametric index (API) for each device n:
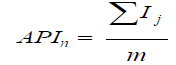
where:
n is a device, for which the calculations are made,
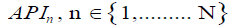
7) Calculation of the average weighted parametric index expressed as a percentage, which reflects the degree of correspondence of each parameter of the assessed product to characteristics of the "ideal" product, taking into account the degree of importance of each characteristic to the consumer:
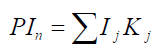
8) Determination of the reduced parametric index (RPIn) of the tested (base) in comparison with the n-th competing device, which provides the comparative assessment of its competitiveness and the comparative value of the product to the consumer. The calculation is made using the following formula:
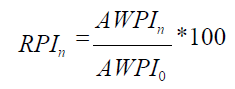
where
AWPI0 is an average weighted parametric index of the base device.
9) Calculation of the markup or discount to the price of the base device (Tn) based on the difference in quality with the n-th competing device:
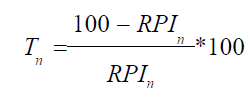
10) Calculation of the indifference price for the base device (Qn) in comparison with the n-th competing device using the following formula:
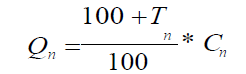
where:
Cn is a price for the competing device.
Thus, the "indifference price" method allows finding out whether a product will be competitive on the market, as well as the price for the product, at which the consumer will be ready to buy it, taking into account the quality level of the product compared to its competitors. The price for the product must correspond to the value of the product for the consumer, which means that the price for the product must be set in accordance with the calculated reduced index, which reflects the relative competitiveness of the product. The admissible markup (discount) to the device due to the difference in the quality of the assessed product and its competitors is calculated on the basis of the above index. At such pricing, the position of the assessed product will be the same as those of competitors, and the customers will be indifferent to the purchase of both products.
Use of this method allows to identify the strengths and weaknesses of the product, which can be taken into account when developing a strategy for its promotion and further R&D aimed at development of the innovative products.
Results
The device competitiveness was assessed and the indifference price was defined for a medical device – express analyzer of critical conditions of myocardial infarction biomarkers, designed by the research team of the Center of Microtechnology and Diagnosis of the SPbSETU. The principles of the device operation are detailed in Zimina T. M., & Luchinin V. V. (2011), (Agreement No. 14.B 37.21. 0793 dated September 03, 2012) (Pat. 2280247 of the Russian Federation, MPK7 G01N21/64, G01N2 /03) (Luchinin V.V., Zimina T.M., 2013).
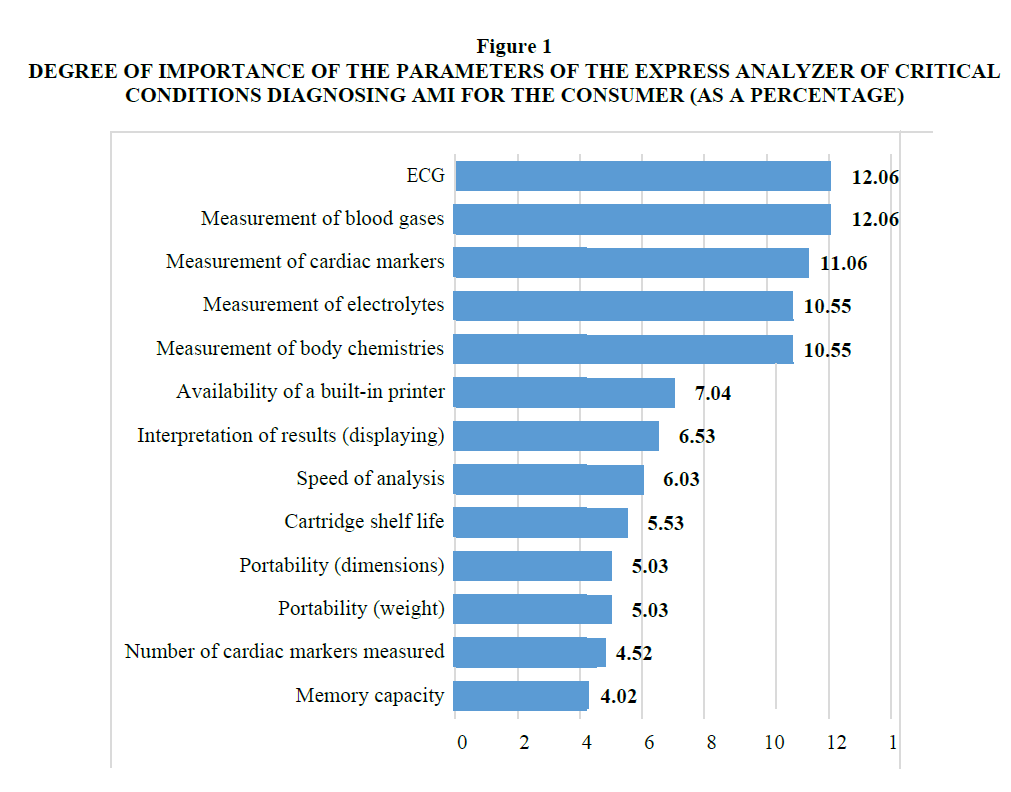
Figure 1.Degree Of Importance Of The Parameters Of The Express Analyzer Of Critical Conditions Diagnosing Ami For The Consumer (As A Percentage).
At the first stage, the market for the express analyzers in Russia was analyzed with the purpose to define the key competitors based on regulatory requirements. As a result, it was determined that the express analyzers of critical conditions belong to the means of preclinical diagnosis, the target segment of which are ambulances equipped in accordance with the Order of the Ministry of Healthcare of the Russian Federation dated 01.12.2005 No. 752 "About equipment of medical ambulances." In accordance with the latter, an express analyzer of critical conditions is a must device for resuscitation trolleys and specialized ambulances. The analysis of this order resulted in identification of two competitors to the domestic device: I-STAT 1-300 (Abbott, USA), which is used to equip ambulances, and ?obas h 232 (Roche, USA). The results of the second stage – comparative analysis of express analyzers of critical conditions – are shown in Table 1.
| Table 1: Comparative Analysis Of The Express Analyzers Of Critical Conditions |
||||
| Characteristics | I-STAT 1 model 300 (Abbott, Point-of-Care, USA) |
?obas h 232 (Roche, Point-of- Care, USA) |
LETI development (Point-of-Care, Russia) |
|
|---|---|---|---|---|
| I | Parameters measured: | |||
| Blood gases | + | - | - | |
| Electrolytes | + | - | - | |
| Body chemistries | + | - | - | |
| Cardiac markers: | ||||
| Troponin I | + | + | + | |
| Creatine kinase-MB | + | + | + | |
| BNP | + | + | - | |
| Myoglobin | - | + | + | |
| II | Shelf life of cartridges/ dipsticks, months |
6 months at a temperature of 2 to 8ºC. Two weeks at a temperature of 18 to 30ºC |
6 months at a temperature of +2°C to +8°C. Up to 1 week at a temperature of +15°C to +25°C |
12 months at a temperature of 2 to 8ºC |
| III | Time required for analysis of cardiac markers |
10 minutes | 15 minutes | 10 minutes |
| IV | Display of information |
LCD display, option to connect to a computer, built-in printer |
LCD display, option to connect to a computer, built-in printer |
Monitor, on-board computer, no built-in printer designed so far |
| V | Dimensions: | |||
| Dimensions, cm | 7.69 x 23.48 x 7.24 | 27.5 x 10.2 x 5.5 | 15 x 10 x 20 | |
| Weight, kg | 0.65 | 0.65 | 0.8 | |
| VI | Customer support in Russia |
well-developed across the country |
well developed across the country |
absent at the moment |
| VII | Registration in the Russian Federation |
yes (Registration Certificate FS No. 2006/807 dated 30.05.2006) |
yes (Registration Certificate FS 2007/00474 dated 23.10.2007) |
absent at the moment |
| VIII | Price | $9,600 + $450 per pack (25 cartridges) |
$8,800 + $230 per pack (10 dipsticks) |
not on sale at the moment |
At the fifth stage, the correspondence of devices to consumer demands was assessed, and the average weighted parametric index was calculated at the sixth stage (Table 2).
| Table 2: Calculation of the Average Parametric Index Of Express Analyzers of Critical Conditions |
|||||
| No. | Specifications | Parameters of the "ideal" device |
Development of SPB SETU "LETI" 1 (Point-of- Care, Russia) |
I-STAT 1- 300 (Abbott, Point-of- Care, USA) |
?obash 232 (Roche, Point of- Care, USA) |
|---|---|---|---|---|---|
| 1 | ECG | yes | 0.00 | 0.00 | 0.00 |
| 2 | Measurement of blood gases | yes | 0.00 | 100.00 | 0.00 |
| 3 | Measurement of cardiac markers |
yes | 100.00 | 100.00 | 100.00 |
| 4 | Measurement of electrolytes | yes | 0.00 | 100.00 | 0.00 |
| 5 | Measurement of body chemistries |
yes | 0.00 | 100.00 | 0.00 |
| 6 | Availability of a built-in printer |
yes | 0.00 | 100.00 | 100.00 |
| 7 | Interpretation of results (displaying each parameter) |
yes | 100.00 | 100.00 | 100.00 |
| 8 | Speed of analysis | 10 min. | 100.00 | 66 | 100.00 |
| 9 | Cartridge shelf life | 12 months | 100.00 | 50.00 | 50.00 |
| 10 | Portability (weight) | 0,65 | 77.00 | 50.00 | 50.00 |
| 11 | Portability (dimensions) | 7.69 x 23.48 x 7.24 |
75.00 | 100.00 | 70.00 |
| 12 | Number of cardiac markers measured (myoglobin was added) |
3 | 100.00 | 90.00 | 100.00 |
| 13 | Memory capacity | 5000 | 80.00 | 100.00 | 90.00 |
| Total | 56.31 | 81.23 | 58.46 | ||
At the seventh stage, based on the weights assigned to each characteristic of the device, the average weighted parametric index was calculated using formula 4, which reflects the degree of correspondence of the compared devices to consumer preferences (Table 3).
| Table 3: Calculation Of The Average Weighted Parametric Index Of Express Analyzers Of Critical Conditions |
|||||
| No. | Specifications | Weighted index,% |
Development of SPB SETU "LETI" 1 (Point-of- Care, Russia) |
I-STAT 1- 300 (Abbott, Point-of- Care |
?obash 232 (Roche, Point of- Care, USA) |
|---|---|---|---|---|---|
| 1 | ECG | 12.06 | 0.00 | 0.00 | 0.00 |
| 2 | Measurement of blood gases | 12.06 | 0.00 | 12.06 | 0.00 |
| 3 | Measurement of cardiac markers |
11.06 | 11.06 | 11.06 | 11.06 |
| 4 | Measurement of electrolytes | 10.55 | 0.00 | 10.55 | 0.00 |
| 5 | Measurement of body chemistries |
10.55 | 0.00 | 10.55 | 0.00 |
| 6 | Availability of a built-in printer |
7.04 | 0.00 | 7.04 | 7.04 |
| 7 | Interpretation of results (displaying each parameter) |
6.53 | 6.53 | 6.53 | 6.53 |
| 8 | Speed of analysis | 6.03 | 6.03 | 3.98 | 6.03 |
| 9 | Cartridge shelf life | 5.53 | 5.53 | 2.76 | 2.76 |
| 10 | Portability (weight) | 5.03 | 3.87 | 2.51 | 2.51 |
| 11 | Portability (dimensions) | 5.03 | 3.77 | 5.03 | 3.52 |
| 12 | Number of cardiac markers measured (myoglobin was added) |
4.52 | 4.52 | 4.07 | 4.52 |
| 13 | Memory capacity | 4.02 | 3.22 | 4.02 | 3.62 |
| Total | 100.00 | 44.52 | 80.16 | 47.59 | |
Thus, the consumer demands are best satisfied by I-STAT 1-300, which by 80% corresponds to the "ideal" representations of the consumer. The domestic express analyzer is currently inferior to its competitors and corresponds to the "ideal" representations of the consumer only by 45%.
At the eighth stage, the reduced parametric indexes of the domestic express analyzer were calculated in comparison with two competitors; discounts to competitors’ prices were calculated at the ninth stage; the indifference prices were determined according to the level of its competitiveness at the last stage (Table 4).
| Table 4: Calculation Of The Indifference Price For The Express Analyzer Of The First Generation | ||
| Indicator | I-STAT 1-300 (Abbott, Point-of-Care, USA) | ?obas h 232 (Roche, Point-of-Care, USA) |
|---|---|---|
| Market prices of competitors, thous. rub. | 780 | 610 |
| Discount to the price of the analyzed device based on difference in quality,% | -44.46 | -6.44 |
| Indifference prices in relation tocompeting products, thous. rub. | 433 | 571 |
Comparison of the domestic device with the competitors allowed to determine that the quality of characteristics of the device I-STAT 1-300 are higher than that of the domestic express analyzer by 44.46%, while the indifference price for the device developed at SPbSETU "LETI" may amount to 433,220 rub. With respect to the I-STAT 1-300.
The study resulted in a conclusion that the project of the domestic express analyzer does not fully correspond to consumer preferences at the moment due to the lack of three groups of measured parameters which the specialists require in their daily work (see Table 1), as well as a built-in printer to print out the results to attach to the case-record (Kozlova E.A., Kudryavtseva T.Yu. 2016). As such, the decision was made to improve the device, taking into account the market demands on the following parameters: availability of a built-in printer, possibility of measuring ECG and general clinical minimum.
| Table 5: Calculation Of The Indifference Price For The Express Analyzer Of The Second Generation | ||
| Indicator | I-STAT 1-300 (Abbott, Point-of- Care, USA) | ?obas h 232 (Roche, Point-of- Care, USA) |
|---|---|---|
| Market prices of competitors, thous. rub. | 780 | 610 |
| Discount to the price of the analyzed device based on difference in quality,% | -7.47 | 55.86 |
| Indifference prices in relation to competing products, thous. rub. | 722 | 951 |
Repeated calculations were made when comparing the improved device – let it be the device of the second generation – with the competitors. It was determined that the quality of ISTAT 1-300 is higher than that of the domestic express analyzer by 7.47%, while the quality of ?obas h 232 is inferior to the analyzed device by 55.86%. Accordingly, the price for the domestic express analyzer with respect to the I-STAT 1-300 may amount to 721,720 rub. (Table 5). Upon the improvement of the device in accordance with the market demands, its position will improve with respect to competing products, and the price will rise accordingly.
Discusssion
The analysis of the market for express analyzers of critical conditions diagnosing AMI and comparisons of consumer characteristics of the analogues of the device developed by researchers at the SPbSETU "LETI" that exist on the market allowed to reveal that it was inferior to competitors by a number of factors at the moment. The key areas of improvements are the expansion of the measured parameters with the ability to analyze the general clinical minimum, integration of a built-in printer and an electrocardiograph, which will be an innovative component of the device that will allow to carry out a comprehensive diagnosis of AMI. As such, the optimal price for the development of the domestic express analyzer of the first and the second – modified – generation of the device was calculated to make the device much-in-demand for consumers, taking into account the calculated competitiveness ratio. The difference in the price for two generations of the device is determined by a different set of consumer characteristics: in particular, the indifference price for the domestic express analyzer of the first generation is from 433 thous. rub. to 571 thous. rub., for the device of the second generation – from 722 thous. rub. to 951 thous. rub.
The conducted study allowed to test the hypotheses of the researchers concerning the correspondence of the designed characteristics of the device to the key demand parameters. The reduced indifference prices are guidelines for determining the future price of the device. Theoretically, it should be not higher than the values obtained for each configuration. Otherwise, the device will be uncompetitive by the price parameter to foreign counterparts.
It should be noted that the optimal market price for the express analyzer under development must be calculated at all stages of the device development, because technical and consumer characteristics of the device, as well as its manufacturing technology, can be adjusted at any stage of development, resulting in the change of its competitive position on the market and, accordingly, the price of the device requires an adjustment in accordance with the newdata.
Conclusion
As such, this study presents the results of testing the individual elements of the organizational and methodical complex under development, which are associated with the assessment of the competitiveness of medical diagnostic devices. The obtained results and recommendations allowed the researchers to obtain information about the key areas of improvement of the device and the demand parameters required to enter the creation stage. Besides, the work on the informational and methodological support of the process of commercialization of the device for express diagnosis of disease biomarkers allowed us to obtain a practical result of the use of the organizational and methodical complex and to implement the processes of development and tool testing at the same time. The qualitative component of the process of developing the tools for commercialization of innovative devices was improved thanks to the principle of continuous learning and obtaining relevant information from the key stakeholders.
Further research involves developing the conceptual provisions and methods based on them, which will allow to assess the potential of introduction and commercialization of innovative diagnostic medical devices, and to effectively manage the introduction and commercialization in the case of a positive forecast. This complex will largely base on foreign research and development in order to support the process of integration as part of the topic of the domestic academic and practical fields under study into the international one, as well as implementation of the import process and adaptation of the best developments to Russian conditions.
Acknowledgements
The article is prepared with the support of the Ministry of Science and Education of the Russian Federation (project No. 26.6446.2017/??).
References
- Alexander, K. & Clarkson, P.J. (2002). A Validation Model for the Medical Devices Industry. Journal of Engineering Design, 13(3), 197-204.
- Imelli, P. & Kobe, C. (2008). Guidelines for the Project Initialization Phase of Medical Device Development. Zurich: Autonomous System Lab, pp: 25.
- Rochford, L. & Rudelius, W. (1997). New Product Development Process: Stages and Successes in the Medical Products Industry. Industrial Marketing Management, 26(1), 67-84.
- Medina, L.A., Kremer, G.E.O. & Wysk, R.A. (2013). Supporting Medical Device Development: A Standard ProductDesign Process Model. Journal of Engineering Design, 24(2), 83-119.
- Mehta, S.S. (2008). Commercializing Successful Biomedical Technologies: Basic Principles for the Development ofDrugs, Diagnostics and Devices. Cambridge University Press, pp: 335.
- Panescu, D. (2008). Medical Device Development. In Conference Proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual Conference, 5591-5594.
- Santos, I.C. (2013). Product Development Methodologies: The Case of Medical Devices. Doctoral Dissertation, Universidade do Porto.
- Shah, S.G.S. & Robinson, I. (2008). Medical Device Technologies: Who is the User?. International Journal of Healthcare Technology and Management, 9(2): 181-197.
- Wood, B. (1996). Characterizing Medical Device Development. Northcon/96, 254-259.
- Philips Plastic Corporation, How to Maximize Speed and Efficiency of Medical Product Development During Pilot Phases and Clinical Trials. Philips Plastic Corporation. Date Views20.05.2016 www.phillipsplastics.com/sites/default/files/whitepaper/WhitePaper_Medical.pdf, last access 11-10-2013.
- Rodionov, D.G., Fersman, N.G. & Kushneva, O.A. (2016) Russian Universities: Towards Ambitious Goals. International Journal of Environmental and Science Education, 11(8), 2207-2222.
- Rodionov, D.G., Rudskaia, I.A. & Kushneva, O.A. ( 2014). The Importance of the University World Rankings in the Context of Globalization. Life Science Journal, 11(10), 442-446.
- Rodionov, D.G., Rudskaia, I.A. & Alexandrovna, K.O. (2014). How Key Russian Universities Advance to Become Leaders of Worldwide Education: Problem Analysis and Solving. World Applied Sciences Journal, 31(6), 1082-1089.
- Zimina, T.M. & Luchinin, V.V. (2011) Microsystems for Express Analysis. Journal of Analytical Chemistry, 66(12), 1136-1158.
- Gorodnikova, N.V., Gokhberg, L.M., Ditkovskiy, K.A. (2016). Indikatory nauki [Indicators of Science].Statistical Yearbook. National Research University "Higher School of Economics", pp: 304.
- NTTS “MEDITEKS” podvel itogi razvitiya rynka meditsinskikh izdeliy v 2014 godu [RDC "MEDITEKS" Summarized the Development of the Market for Medical Products in 2014]. Remedium. Date Views 20.11.2015 www.remedium.ru/news/relis/detail.php?ID=64791.
- Order dated January 31, 2013 No. 118 "Ob utverzhdenii strategii razvitiya meditsinskoy promyshlennosti rossiyskoy federatsii na period do 2020 goda [About Approval of the Strategy of Development of Medical Industry of the Russian Federation for the Period through to 2020]. Date Views 14.06.2016 www.consultant.ru/document/cons_doc_LAW_145833.
- Federal Target Program "Razvitiye farmatsevticheskoy i meditsinskoy promyshlennosti Rossiyskoy Federatsii na period do 2020 goda i dalneyshuyu perspektivu [Development of the Pharmaceutical and Medical Industry of the Russian Federation for the Period through to 2020 and Further Prospects] (Approved by the Decree of the Government of the Russian Federation dated February 17, 2011 No. 91).
- Zimina, T.M. & Luchinin, V.V. Russian Federation Patent 2280247, MPK7 G01N21/64, G01N21/03. Biochip dlya flyuorestsentnogo i lyuminestsentnogo analiza [Bio-chip for Fluorescent and Luminescent Analysis].
- Luchinin, V.V & Zimina, T.M. (2013). Reasearch and Technical Report “Razrabotka fiziko -khimicheskikh osnov i sozdaniye obraztsa analiticheskoy mikro-nanobiosistemy dlya doklinicheskoy ekspress identifikatsii biomarkerov OIM (etap 2, itogovyy) [Development of Physicochemical Bases and Creation of the Sample of the Analytical Micro-Nanobiosystem for Preclinical Express Identification of AMI biomarkers (step 2, final)]. Saint - Petersburg: SPbSETU "LETI", pp: 10-14.
- Lifits, I.M. (2014). Formirovaniye i otsenka konkurentosposobnosti tovarov i uslug [Formation and Assessment of Competitiveness of Goods and Services]. Moscow: Uright, pp: 448.