Research Article: 2021 Vol: 25 Issue: 2
Loud and Unclear-An Exploratory Empirical Vocal Analysis of Risks versus Rewards in Direct-To-Consumer Television Advertisements
Vivek S. Natarajan, Lamar University
Nandhu Radhakrishnan, Lamar University
Kabir C. Sen, Lamar University
Kathleen Broussard, Texas A&M University
Abstract
Little research has been performed on the vocal presentation of risks and benefits in DTC prescription drug advertisements. We analyzed the effects of the vocal presentations of negative side effects and benefits in prescription drug Direct-to-Consumer advertising. We also analyzed the rate of speech, pitch range of speech, duration spent, and quotients of risk information compared to benefit information, listed in DTC ads. The data was examined to see if the severity of the ailment and/or the date the ad was released impacts the vocal presentation of side effects. Contrary to expectations, more time was allocated to stating the risks in comparison to the benefits. However, the rate of speech was faster during the delivery of risks involved. These results suggest the need for standardization in rate of speech for advertisements such that both benefits and risks of medication are perceived equally by the audience.
Keywords
Direct to Consumer Advertisements, Vocal Analysis, Risks, Rewards, Television Advertising.
Introduction
Direct to consumer advertisements of prescription medications are focused on appealing to the needs of customers. Unlike the days when drugs were only marketed to physicians, now direct to consumer advertisements provides average people with the awareness of the various medications available on the market. Armed with the knowledge of treatment options, patients now can influence what medications their physicians prescribe to them. There is a great deal of controversy that surrounds how much influence direct to consumer advertising has over pushing certain treatments. Currently, the United States and New Zealand are the only two developed countries that allow pharmaceutical companies to advertise directly to consumers (Bell et al., 2000). The U.S. government has set detailed regulation about how information about prescription medications are presented to the public (Mogull, 2008).
The purpose of this research is to analyze the vocal communication of risks versus benefits in Direct-to-Consumer advertisements of prescription drugs. We compare the differences between the vocal presentations of the drug risks compared to the presentation of drug benefits in DTC commercials. DTC commercials, ranging from the year 2000 to 2014. The paper is organized as follows. We discuss the debate, the background, and the regulations for direct to consumer advertising. We then present a summary of the existing literature over direct to consumer advertising. We then discuss the methodology and the results. We conclude with a discussion of results and implication.
Direct-to-consumer advertising (DTCA) is a controversial subject (Auton, 2004; Chandra & Miller, 2005; Folsom et al., 2010; Frosch et al., 2010; Mukherjee et al., 2013). Those who support DTCA believe that by directing pharmaceutical advertisements specifically at consumers it allows the public to become more informed of potential health concerns and medical treatments. Alternatively, those who oppose DTCA think that by advertising prescription drugs directly to consumers, pharmaceutical companies are harming the physician and patient relationship and manipulating healthy individuals to make requests for unnecessary drugs.
Debate
Currently, the United States and New Zealand are the only two developed countries that allow pharmaceutical companies to advertise directly to consumers (Block, 2007). Direct-to-consumer advertising (DTCA) is a controversial subject. Those who support DTCA believe that by directing pharmaceutical advertisements directly at consumers it allows the public to become more informed of potential health concerns and medical treatments. Alternatively, those who oppose DTCA think that by advertising prescription drugs directly to consumers, pharmaceutical companies are harming the physician/patient relationship and manipulating healthy individuals to request unnecessary drugs. This summary will provide a brief regulatory background and an outline of the major arguments for and against DTCA.
Pros
DTCA allows consumers to have more control over their health. The Internet, TV and print advertisements for prescription drugs provide consumers, who otherwise might not have access to medical information, with the knowledge of the latest health treatment options (Auton, 2006). DTCA encourages consumers to meet with their physicians. A 2004 study by the FDA showed that DTCA prompted 27% of the U.S. population to meet with a physician to seek more information about an existing ailment (Ventola, 2011). The prescription medication ads increase communication between patients and their physicians. Informed viewers of DTCA are given the confidence to ask more instructive questions regarding their health issues. DTCA is known to lead to better patient compliance. According to a study conducted by the FDA in 2003, 54% of doctors believed that “DTCA improved patient compliance by increasing the probability of the patient taking their medicine properly”. The pharmaceutical advertisements create awareness of rare or uncommon health conditions. For instance, before Procrit, a medication that treats anemia, was advertised, medication to prevent fatigue was rarely prescribed by doctors because people did not discuss their symptoms of fatigue with their physicians. After the Procrit campaign aired anemic chemotherapy cancer patients realized they could combat their symptoms of fatigue by requesting to take the medication Procrit (Ventola, 2011). Effective DTCA improve patient welfare by producing a more health conscious society, enhancing the doctor/patient relationship, and decreasing the number of under-diagnosed patients.
Cons
The average consumer does not have a medical background to help them interpret DTCA. DTCA can easily confuse and mislead consumers into having an incorrect impression of the effectiveness of a drug. Pharmaceutical companies must only list the drug’s major risk factors. The open interpretation of the term “major” risk factors enables a DTC ad to have an uneven list of risks and benefits. By withholding side effects and using vague descriptions, pharmaceutical companies could use DTCA to exaggerate the benefits of a drug (Ventola, 2011). Often DTC ads
rely more on the use of emotional appeals than educating consumers about the medical condition and risks factors. Research shows that 95% of prescription drugs use emotional appeals in ads (Frosch et al., 2007). Instead of educating consumers, DTC advertisements use emotional appeals to convince people that a medication will improve consumers’ lives. For example, not only do DTC ads portray medications as a cure to medical conditions, but it also portrays medication as a way for alleviating fear, calming distress, and gaining social approval for patients. DTCA promote inappropriate pharmaceutical drugs by exaggerating benefits, withholding pertinent information, and using misleading emotional appeals.
Brief Background
In 1969, the Food and Drug Administration (FDA) composed the first set of restrictions regarding DTCA, which required each prescription drug ad to contain a balanced account of all risks and benefits, a complete list of all side effects, and comply with formatting criteria. The amount of details demanded by the FDA made it difficult to promote medications through standard media channels. The facts could not be easily presented in the average length of a TV commercial or in the limited space of a one-page print ad. The pharmaceutical industry argued that the regulations put on DTCA prevented it from “educating” the public of medical treatment options. In August of 1997, the FDA released a temporary guidance that reduced the number of requirements for DTC broadcast advertising. Then in August of 1999 the FDA finalized the “Industry Guidance on Consumer Directed Broadcast Advertisements.” From that point forward prescription advertisements only had to list the major side effects and give a location of supplementary information (Hartgraves, 2002).
DTC Regulations
The Food, Drug, and Cosmetic Act regulates direct to consumer drug advertisements. It is the responsibility of the U.S. Food and Drug Administration (2012, 2013) to monitor and enforce regulations on DTC advertisements. The FDA requires that DTC advertisements present a “fair balance” of the risks and benefits of prescription drugs. There are specific requirements set for print and broadcast direct to consumer advertisements. Print direct to consumer ads must contain a “brief summary” about the drug. The FDA defines a “brief summary” as a comprehensive explanation of the drugs positive and negative side effects. A “brief summary” should contain who should avoid taking the drug, when should someone discontinue using the drug, and what are the severe and routine side effects associated with the drug. Direct to consumer print ads usually have one whole page devoted just to the brief summary. Since the average television and radio commercial does not have the time available to present the extensive amount of information required in a brief summary, the FDA created the “Guidance for Industry Consumer–Directed Broadcast Advertisements.” The guidance stipulates what information must be disclosed in prescription human and animal drug broadcast advertisements. Direct to Consumer ads aired on television, radio, and telephone communications systems must provide the “major statements” and make “adequate provisions” about the prescription drug. The “Major Statements” consists of the presentation of drug’s most important risks and side effects. This information must be included in the audio and can be accompanied by a visual in a television commercial. There is no exact minimum or maximum set for the “major statements.” The amount of risk information that must be included depends on the medication being advertised. Since the complete information about a drug cannot be relayed in a short television or radio ad, “Adequate Provision” must be provided. “Adequate Provisions” redirects viewers to where they can locate additional information about the drug. These provisions can be met by providing a web address, giving a toll-free telephone number, directing viewers to look at a specific print ad, and recommending that they consult a physician for question about the medication (FDA, 2012).
Mandatory Disclosures
To protect the public, the federal government requires “fair balance” disclosures of prescription drug side effects and benefits. Most industries are not required to mention the bad qualities of a product in its advertising. The government orders mandatory disclaimers for products that are harmful to the health of people. For instance, in the United States there is a mandatory Surgeon General Warning placed on all tobacco products. In the following section there will be a discussion of the history of the mandatory disclaimers put on tobacco products.
Tobacco
The U.S. federal government regulates cigarettes and smokeless tobacco advertising. On January 11, 1964, Luther L. Terry, M.D., Surgeon General of the U.S. Public Health Service, released a Report on Smoking and Health (Center of Disease Control and Prevention, 2009, 2012). For the first time the fact that smoking cigarettes can cause lung cancer was acknowledged. This revelation led to Congress enacting the Federal Cigarette Labeling and Advertising Act of 1965. The act required that all cigarette packaging and advertising have warning labels explaining that cigarettes are harmful to people’s health. It was mandatory that the label read "Caution: Cigarette Smoking May Be Hazardous to Your Health" somewhere on the package or ad. Later congress made alterations to wording of the health warning should read. The Public Health Cigarette Smoking Act of 1969 mandated that the warning label should say “Warning: The Surgeon General Has determined that Cigarette Smoking Is Dangerous to Your Health” The act also banned cigarette advertising on television and radio. In 1984, congress passed the Comprehensive Smoking Education Act, which created a mandatory rotating health warnings system for all cigarette packaging and advertisements.
Cigarettes warning labels had to rotate through four different health warning every three months. The four updated warning labels consisted of the following: smoking causes lung cancer, heart disease and may complicate pregnancy; quitting smoking now greatly reduces serious rinks to your health; smoking by pregnant women may result in fetal injury, premature birth, and low birth weight; cigarette smoke contains carbon monoxide (CDC, 2012). Advertising for Smokeless tobacco has also been limited with regulations. In 1986, congress passed the Comprehensive Smokeless Tobacco Health Education Act. The act banned smokeless tobacco advertising on broadcast media and established three mandatory rotating warning labels. All smokeless tobacco packaging and advertisements were required to have one of the three following warning labels: this product may cause mouth cancer; this product may cause gum disease and tooth loss; and this product is not a safe alternative to cigarettes.
The cigarette and Smokeless tobacco industry are continually being met with new limitations and requirements. Most recently, the Family Smoking Prevention and Tobacco Control Act was made law on June 22, 2009. The Tobacco Control Act requires the cigarette and smokeless tobacco packaging to have larger warning labels, additional warnings, and add visual images that warn smokers of the side effects of smoking. The warning labels must have larger font sizes. There will be none rotating warning messages that must be rotated. The warning messages will have images displaying the negative side effects of smoking. For example, a message that read “Cigarettes cause fatal lung disease” will be accompanied by a photo of an unhealthy lung. (FDA, 2013)
Extant Literature on Presentation of Risks in DTC Ads
There have been numerous research papers over the effectiveness of direct to consumer advertisements. The FDA requires that direct to consumer advertisements contain a “fair balance” presentation of the prescription drug side effects and benefits. A fair balance presentation requirement restrains advertisers from focusing attention on the benefits and understating side effects. Numerous content analyses have been conducted to assess the comparability of the presentation of side effects of benefits in direct to consumer advertising. The following section will summarize existing literature over communication formats and the validity of the “fair balance” presentation in direct to consumer advertisements.
Professor Joel Davis, from San Diego State University, conducted an experiment to see how the organization of risk and benefits in direct to consumer ads can alter how safe or effective a drug is regarded. The experiment entailed the creation of three fake prescription drugs that would treat three common illnesses. The numbers, the order, and the mode of the presentation of the side effects were adjusted to test how those factors influenced the customers’ opinions on the severity of the side effects. The study concluded that the number of side effects and the mode of presentation influenced the customer reactions. For instance, the more side effects listed, the less safe the drug was regarded. Customers have a more positive attitude towards direct to consumer ads with oral communication of side effects compared to print. The order of the side effects in the list does not impact how customers judge the safety of a drug. However, the severity of a side effect impacts the customer’s opinion of how often a side effect will takes place. Majority of the respondents believed that the more severe the side effect, the less likely it would occur (Davis & Meader, 2009).
Thorsen (2009) conducted a study to evaluate whether the presentation of risk and benefit information in direct to consumer broadcast advertisements followed the fair balance guidelines set by the FDA. He examined factors such as the number of statements assigned, the pace of the audio, the amount of time allotted, and the quantity of space given to the presentation of the risks and benefits. Direct to consumer advertisements that aired during the nightly news over a period of one month were collected from two different television networks. These samples were then analyzed. The data gathered showed that the number of risk statements did not equal benefit statements. All benefit statements and majority of the risk information were said at a steady normal pace. More time was allotted to the risk information than to the benefit information. The benefit information was shown in print larger and a more central location compared to the risk information (Thorsen, 2009). This research represents that direct to consumer advertisements do not always have a balanced presentation of warnings and benefits. The time, speed, space, and location all factor into how fairly information is presented.
This leads to the motivation for our study. There has been no study of an analysis of the vocal presentation of direct to consumer television advertisements till date in the literature. Our study aims to fill this important gap. We analyze the vocal communication of risks versus benefits in Direct-to-Consumer advertisements of prescription drugs. We compare the differences between the vocal presentations of the drug risks compared to the presentation of drug benefits in DTC commercials. DTC commercials, ranging from the year 2000 to 2014. We then discuss the results (Wilkes et al., 2000).
Methodology
We observed a sample of DTC advertisements and gained an understanding of the components of DTC commercials. Then, we exported DTC TV ads from the internet using media software and converted the videos into audio files. We identified the Benefit and Risk messages contained in the voice track. The time duration of benefits and risks were documented. Then, a voice analysis of the advertisement during the benefits and risks was done. We employed state of the art procedures, described under “Data Collection”, to measure the rate of speech, pitch range, duration, and quotient of the presentation of risk information compared to benefit information in each DTC advertisement. Then we examined the data collected and tested it for any relationships between the vocal presentation of the side effects of the drugs and the years the ads were produced and/or the severity of the ailments the drugs can treat.
Goal
We categorized the DTC commercials according to the severity of the ailment that the drug that is being advertised can treat (i.e. lifestyle drugs).
Benefits
A benefit is help provided by a drug for the person who is taking it. The law does not allow drug companies to advertise benefits unless they are related to the FDA-approved use. Benefits of prescription drugs can be used to control symptoms of difficult medical conditions, often pertaining to rare diseases, as well as medical concerns related to lifestyle segments and situations.
Risks
Risk covers information that answers the following issues/-What groups should not use the drug, when the drug should not be used, Serious and commonly occurring side effects, Side effects seen in special populations, the chance of dependency, the chance of, and withdrawal effects.
Expected Results
We hypothesize that the data will show that most DTC ads will emphasize the benefits of the medications by pronouncing the positive effects more clearly and at a higher volume compared to the risks of the medications. The benefits of the drugs will be said at a lower rate of speech, at a higher pitch, and at a higher vocal intensity. In contrast, the negative side effects will be spoken at a faster rate of speech, lower pitch, and lower vocal intensity. We predict that the severity of the ailment the prescription drug can treat will influence how the side effects are stated in a DTC commercial. The less life threatening the health concern, the faster and the quieter the negative side effects will be listed in a DTC add.
Data Collection
Speech production involves an intricate balance of systems and subsystems of our body. The audible output goes through a series of kinematic, aerodynamic, and acoustic modulations.
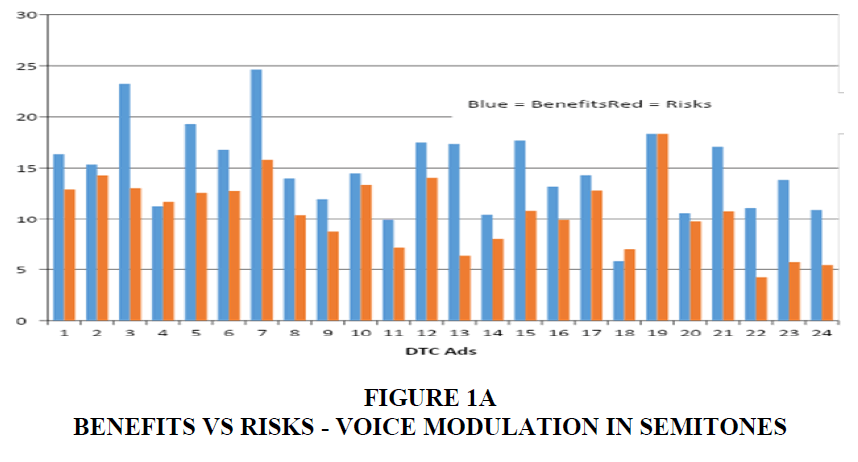
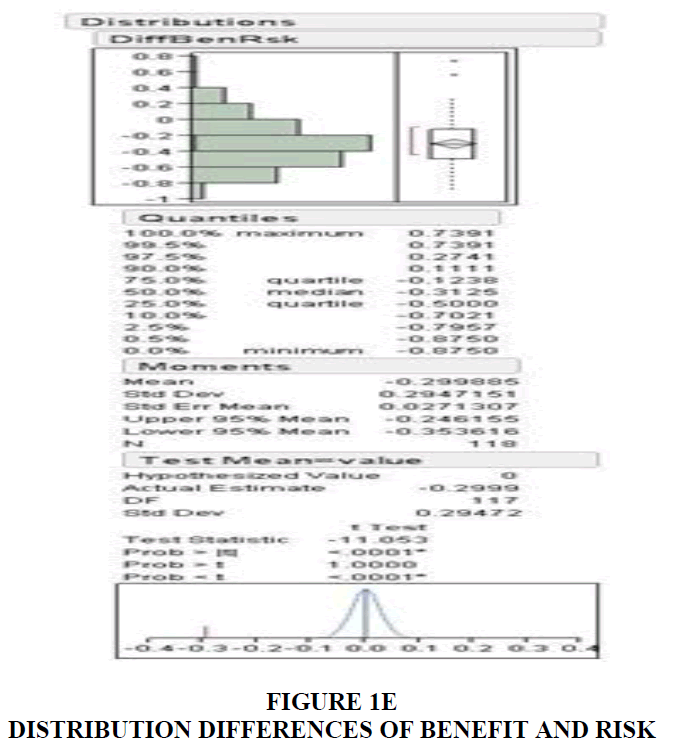
Speakers convey their thought in a series of phrases that is relevant to the context involved and listeners interpret what they hear based on the semantics and syntax that was intended by the speaker. However, speakers can draw the attention of their audience based on how they manipulate their spoken utterance. The emotional component of speech can be influenced by several features. Johnstone & Scherer (2000) compiled acoustic features that reflected common emotions. Speech intensity, fundamental frequency, frequency variability, frequency range, sentence contour, and rate of speech were some of them. Intensity and frequency are the physical correlates of loudness and pitch. Frequency variability indicates pitch modulation which is inferred by plotting the sentence (pitch) contour and measuring pitch range across any given speech sample. Pitch range can be expressed in Hertz (Hz) as the difference between the highest and lowest frequency locations shown on a sentence contour. However, expressing this measure in semitones (ST) may be more accurate. For example, the range between 100 Hz and 200 Hz is the same as that between 1000 Hz and 2000 Hz because both 200 Hz and 2000 Hz are one octave (12 ST) away from 100 Hz and 1000 Hz, respectively. Rate of speech refers to the number of syllables uttered by the speaker in a given time, for instance, per second. Voice and speech analysis have become easier with technological advancement. Duration of risks and benefits were calculated by directly counting the number of seconds these contents were delivered. Based on this number and the duration of the ad, we have derived quotients for benefits and risks. Benefit Quotient is the ratio between the number of seconds spent for benefits divided by the total duration of the ad. Risk Quotient was similarly derived. The current study analyzed rate of speech in syllables per second, pitch range in semitones, benefit and risk time in seconds, and benefit and risk quotient in percentage (Figure 1A-E).
Methodology for Speech Analysis
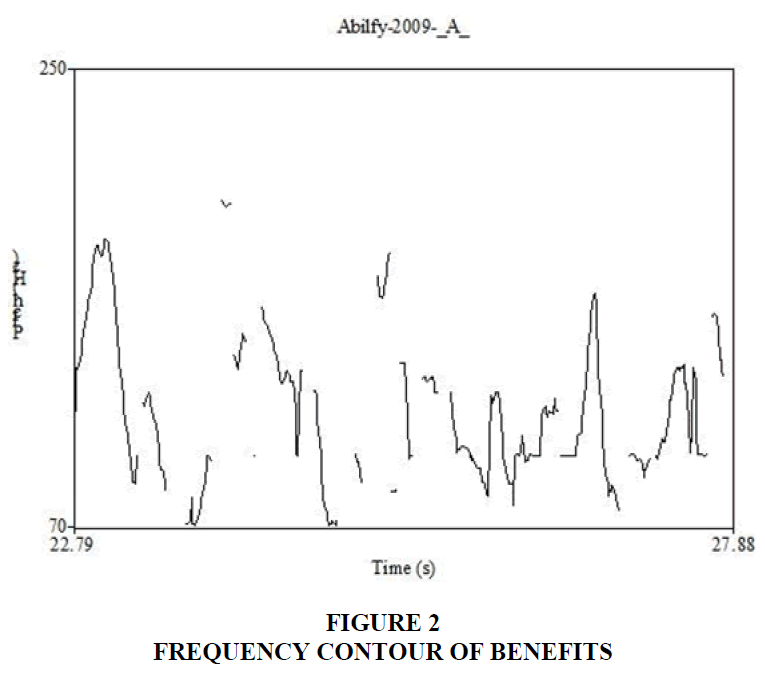
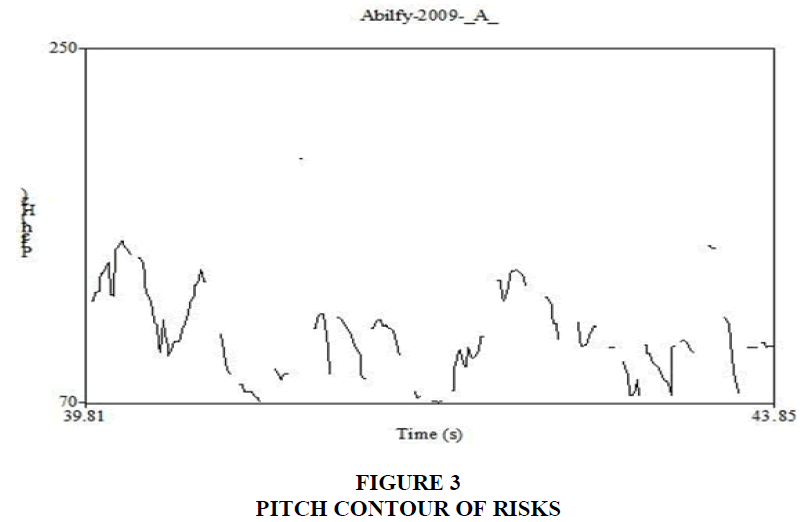
The video files were converted to audio files using RealTimes Convertor, a free program downloaded from RealPlayer®. Based on the content of these audio files, the benefits and risks sections were marked as segments of interest. These segments were analyzed using Praat (version 5.3.56, Boersma & Weenink, 2014), software used for speech analysis. Praat extracts the sentence contour of speech and displays it as a frequency waveform across time. The waveform can be clicked at any location to evaluate the fundamental frequency of the narrator’s voice. Figures 2 and 3 shows the sentence contours of a benefit and risk segment, respectively. The highest and lowest frequencies of each phrase were taken and the pitch range between these two frequencies was calculated in semitones. For example, if the highest frequency is 250 Hz and lowest is 125 Hz, the range in semitones will be 12 ST. The range calculated for phrases within each segment were averaged. Statistical analysis was performed between the mean pitch range calculated for benefits and risks. Results indicate that the pitch range is wider for benefits than risks. This suggests that narrators inflect their speech while discussing benefits. The rate of speech during these two segments, benefits and risks, were also calculated using the same software. These two segments were transcribed in the International Phonetic Alphabet (IPA). Number of syllables per utterance were calculated and divided by the duration in seconds. Results indicate that risks had a greater number of syllables than benefits. This suggests that narrators have a faster rate of speech while discussing risks.
Results
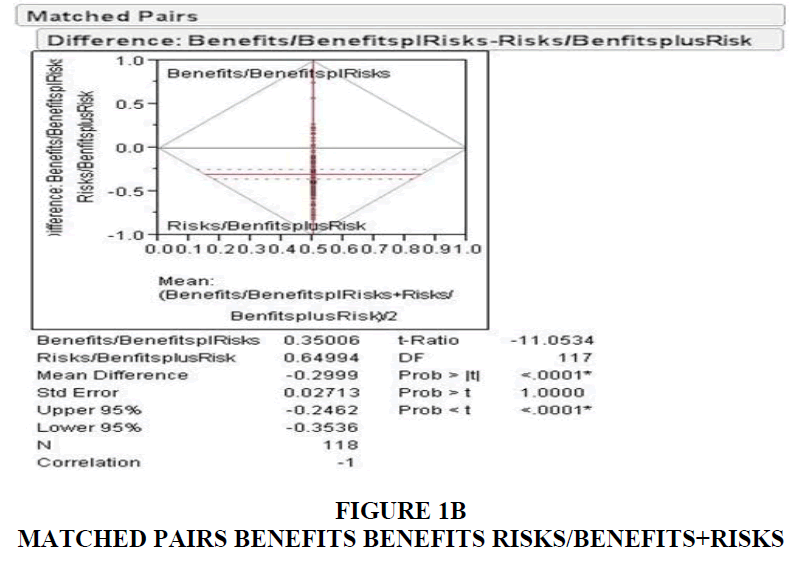
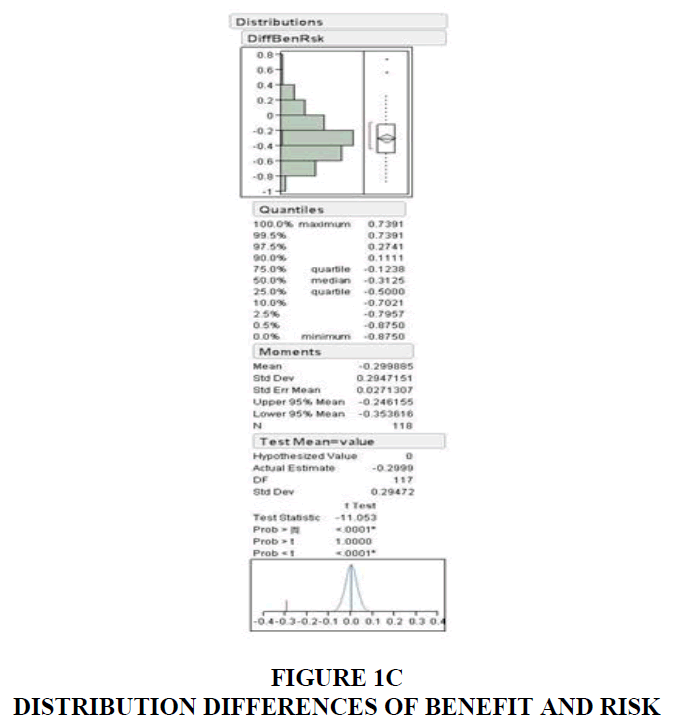
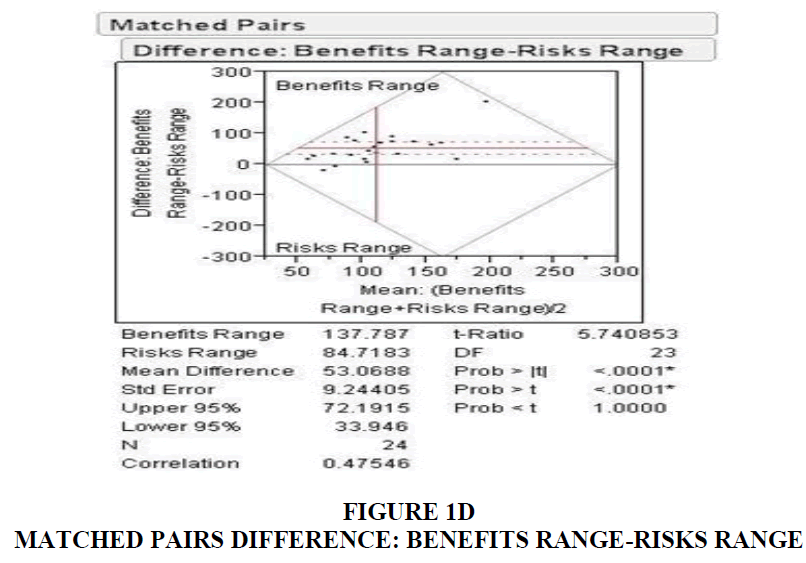
Out of the 118 DTC ads measured, 117 of the ads devoted more time to the presentation of the drug risk information compared to the amount of time devoted to the presentation of the drug benefit information. The average rate of speech used to present the benefit information was 4.39 syllables/second. Whereas the average rate of speech used to present the risk information was 5.44 syllables/second. During pitch range calculation, music composition in most of the ads interfered with the narrator's delivery of benefits and risks. Only 37 ads were free from this hindrance and qualified for this analysis. The pitch range obtained from these ads revealed an average of 13.56 semitones for benefits and 9.13 for risk information.
The duration spent on risks; 22.8 seconds was greater than the time spent for benefits information (11.05). Similarly, the percentage of time spent on benefits and risks, identified as benefit quotient and risk quotient, were 17.75% and 35.08% in Table 1 and Table 2.
| Table 1 Direct to Consumer Advertising Analysis | ||
| Benefits | Risks | |
| Rate of Speech | 4.39 (0.32) | 5.44 (0.28) |
| Pitch Range | 13.56 (4.3) | 9.13 (3.8) |
| Time | 11.05 (5.1) | 22.8 (10.8) |
| Quotient | 17.75 (7.49) | 35.08 (13.22) |
| Table 2 | |||||
| Commercial ID | Name Brand | Treatment | Year | Total Duration( minutes and seconds) | Content Description |
| 1 | Ability | depression | 2009 | 1:15 | Depressed man and woman |
| 2 | Ability | depression | 2010 | 1:15 | Mom walking on a pier with family |
| 3 | Ability | depression | 2011 | 1:30 | Cartoon: Lady that fell into a hole |
| 4 | Ability | depression | 2013 | 1:16 | Cartoon: Lady at office and with family |
| 5 | Aciphex | Acid Reflux | 2010 | 1:00 | Father cooking |
| 6 | Aricept | Alzheimer’s | 2009 | 1:01 | Grandmother with family |
| 7 | Aricept | Alzheimer’s | 2012 | 1:03 | Airport Grandfather |
| 8 | Axiron | male hormone | 2013 | 1:00 | Backyard Birthday Party |
| 9 | Axiron | male hormone | 2013 | 0:59 | Baseball Umpire |
| 10 | Beyaz | birth control | 2011 | 1:14(IC) | Women Shopping |
| 11 | Boniva | osteoporosis | 2013 | 0:59 | Sally Field sitting on a pier |
| 12 | Celebrex | inflammatio n | 2007 | 2:28 | Blue and white cyclist |
| 13 | Celebrex | inflammation | 2008 | 1:00 | Cartoon: Tennis player |
| 14 | Celebrex | inflammation | 2013 | 1:30 | Woman Swimming |
| 15 | Celebrex | inflammation | 2013 | 1:30 | Man walking his dog on the beach |
| 16 | Chantix | stop smoking | 2009 | 2:32 (2:42) | Man reading in his garden |
| 17 | Chantix | stop smoking | 2013 | 1:00 | Father and son |
| 18 | Cialis | erectile dysfunction | 2007 | 1:00 | Parents and college age daughter |
| 19 | Cialis | erectile dysfunction | 2012 | 1:00 | Couple at a restaurant |
| 20 | Cialis | erectile dysfunction | 2013 | 1:00 | Couple playing tennis |
| 21 | Crestor | high cholesterol | 2011 | 1:02 | Man, on staircase |
| 22 | Crestor | high cholesterol | 2011 | 1:00 | Woman walking for exercise |
| 23 | Crestor | high cholesterol | 2012 | 1:02 | Man walking on a boardwalk |
| 24 | Crestor | high cholesterol | 2012 | 1:01 (1:04) | White Woman wearing a purple top |
| 25 | Crestor | high cholesterol | 2012 | 0.59 (1:00) | African American Woman wearing a purple floral top |
| 26 | Crestor | high cholesterol | 2012 | 1:00 | Grandmother with grand kids on the beach |
| 27 | Cymbalta | depression | 2009 | 1:18(1:31) | Walking in the forest |
| 28 | Cymbalta | depression | 2011 | 1:00 | Woman sitting on a couch |
| 29 | Detrol LA | overactive bladder | 2007 | 1:00 (1:20) | Woman in red at a BBQ |
| 30 | Detrol LA | overactive bladder | 2008 | 1:04 | Teacher with a bladder problem |
| 31 | Epiduo | acne | 2013 | 1:00 | Teenagers with acne |
| 32 | Exelon | dementia | 2009 | 0:59 | Daughter writing in journal |
| 33 | Flomax | BPH | 2009 | 1:00 | Men kayaking |
| 34 | Flomax | BPH | 2010 | 1:00 | Men running to the restroom |
| 35 | Humira | rheumatoid arthritis | 2013 | 1:15 | Woman at the Hair Salon |
| 36 | Humira | rheumatoid arthritis | 2013 | 1:14(1:15) | Woman in the kitchen |
| 37 | Humira | rheumatoid arthritis |
2013 | 1:14 | Builder |
| 38 | Imitrex | migraine | 2002 | 1:00 | Nurse in the nursery |
| 39 | Imitrex | migraine | 2002 | 1:01 | Camping Lady |
| 40 | Imitrex | migraine | 2003 | 1:00 | Traffic |
| 41 | Imitrex | migraine | 2003 | 1:00 | Child riding bike |
| 42 | Imitrex | migraine | 2005 | 1:00 | On a date |
| 43 | Januvia | diabetes | 2008 | 0:53(IC) | Walking up stairs |
| 44 | Latisse | eyelashes | 2010 | 1:00 | Blonde lady w/ blue |
| 45 | Latisse | eyelashes | 2011 | 1:00(1:09) | Brooke Shields |
| 46 | Latisse | eyelashes | 2013 | 1:01(1:05) | Mom, business lady |
| 47 | Levemir | diabetes | 2012 | 1:00 | Road trip |
| 48 | Lipitor | high cholesterol | 2004 | 0:59 | Man riding bike |
| 49 | Lipitor | high cholesterol | 2009 | 1:01 | Biking in the woods |
| 50 | Lipitor | high cholesterol | 2010 | 0:59 | Rope over rocks |
| 51 | Lipitor | high cholesterol | 2011 | 0:59(1:04) | Sailing and scuba diving |
| 52 | Lipitor | high cholesterol | 2011 | 1:01 | Skating on thin ice |
| 53 | Lipitor | high cholesterol | 2011 | 0.40(IC) | Couple hiking |
| 54 | Lunesta | insomnia | 2006 | 0.58(1:00) | Butterfly flying around |
| 55 | Lunesta | insomnia | 2011 | 1:00 | Gives you wings |
| 56 | Lunesta | insomnia | 2011 | 0:59 | Hour after hour |
| 57 | Lunesta | insomnia | 2012 | 1:00 | Tossing and turning |
| 58 | Lunesta | insomnia | 2013 | 0:59 | Sleepy worried man |
| 59 | Lyrica | seizures, pain | 2010 | 1:02(1:12) | Fashion Designer |
| 60 | Lyrica | seizures, pain | 2013 | 1:00 | Mother at a carnival |
| 61 | Lyrica | seizures, pain | 2013 | 1:00(1:03) | Grocery shopping |
| 62 | Lyrica | seizures, pain | 2013 | 1:14(1:15) | Man watering garden |
| 63 | Lyrica | seizures, pain | 2013 | 1:00 | Construction woman |
| 64 | Lyrica | seizures, pain | 2013 | 1:15 | Grandma with grandkids |
| 65 | Lyrica | seizures, pain | 2013 | 1:00 | Lady working at a bakery |
| 66 | Mirena | birth control | 2008 | 0:59(1:03) | A lot happens in five yrs. |
| 67 | Mirena | birth control | 2010 | 1:15 | Kids playing in backyard |
| 68 | Nasonex | nasal allergies | 2006 | 0:42 | Bee and an owl |
| 69 | Nasonex | nasal allergies | 2009 | 0.30(0:34) | Won't make you drowsy |
| 70 | Nasonex | nasal allergies | 2009 | 0:30 | A ticking clock |
| 71 | Nasonex | nasal allergies | 2009 | 0:32 | For his sweet |
| 72 | Nasonex | nasal allergies | 2011 | 0:32 | Bee and owl #2 |
| 73 | Nasonex | nasal allergies | 2013 | 0:45(0:58) | Pink flower petals |
| 74 | Nasonex | nasal allergies | 2013 | 0:46 | Allergy seasons |
| 75 | Nasonex | nasal allergies | 2013 | 0:29 | Flower blossom beckons |
| 76 | Nexium | GERD | 2001 | 1:00 | Rocky near the ocean |
| 77 | Nexium | GERD | 2004 | 0:59 | Talking about "better" |
| 78 | Nexium | GERD | 2006 | 1:01 | The "finisher" |
| 79 | Orencia | arthritis | 2008 | 1:00 | Can you grasp this? |
| 80 | Orencia | arthritis | 2013 | 1:00 | I want I can |
| 81 | Paxil | depression | 2000 | 1:00 | Offers new hope |
| 82 | Paxil | depression | 2001 | 1:00 | Talking about worry |
| 83 | Paxil | depression | 2002 | 1:00 | Used to be happy |
| 84 | Plavix | blood clots | 2002 | 1:00 | Grandfather fishing |
| 85 | Plavix | blood clots | 2002 | 0:59 | Better things to do |
| 86 | Plavix | blood clots | 2003 | 1:00 | A million hospitalized |
| 87 | Plavix | blood clots | 2005 | 1:00 | Bill the hockey coach |
| 88 | Plavix | blood clots | 2008 | 1:14 | Waiting to strike |
| 89 | Plavix | blood clots | 2010 | 1:00 | Woman golfer |
| 90 | Pradaxa | Afib, strokes | 2011 | 1:16 | Three doctors |
| 91 | Pradaxa | Afib, strokes | 2014 | 1:15 | Father goes visits doctor |
| 92 | Pristiq | depression | 2010 | 1:15 | |
| 93 | Provenge | cancer | 2013 | 1:00 | |
| 94 | Restasis | dry eyes | 2009 | 0:58 | |
| 95 | Restasis | dry eyes | 2010 | 0:29 | |
| 96 | Restasis | dry eyes | 2010 | 1:01 | |
| 97 | Rozerem | insomnia | 2006 | 1:01 | |
| 98 | Seroquel XR | depression | 2011 | 1:30 | |
| 99 | Spiriva | bronchospasm | 2011 | 1:00 | |
| 100 | Spiriva | bronchospasm | 2011 | 1:00 | |
| 101 | Spiriva | bronchospasm | 2012 | 1:02 | |
| 102 | Spiriva | bronchospasm | 2013 | 1:00 | |
| 103 | Spiriva | bronchospasm | 2013 | 1:00 | |
| 104 | Stelara | Psoriasis | 2010 | 1:00 | |
| 105 | Stelara | Psoriasis | 2013 | 1:00 | |
| 106 | Symbicort | asthma | 2010 | 1:00 | |
| 107 | Symbicort | asthma | 2010 | 1:00 | |
| 108 | Symbicort | asthma | 2011 | 1:01 | Green Truck |
| 109 | Symbicort | asthma | 2011 | 1:00 | Baseball Grandfather |
| 110 | Symbicor t | asthma | 2012 | 0:59 | Fisherman Grandfather |
| 111 | Tamiflu | flu | 2012 | 1:01 | Big man, small house |
| 112 | Vesicare | overactive bladder | 2009 | 0:59 | |
| 113 | Vesicare | overactive bladder | 2010 | 1:00 | |
| 114 | Vesicare | overactive bladder | 2012 | 1:00 | |
| 115 | Vesicare | overactive bladder | 2012 | 1:02 | |
| 116 | Viagra | erectile dysfunction | 2011 | 0:57 | Truck pulled by horses |
| 117 | Viagra | erectile dysfunction | 2012 | 1:02 | Man on a sailboat |
| 118 | Xeljanz | Rheum. arthritis | 2013 | 1:30 | Arms are made for hugs |
Discussion and Conclusion
Contrary to expectations, more time was allocated to stating the risks in comparison to the benefits. However, the rate of speech was faster during the delivery of risks involved. This may have an influence on the listener. Faster speech could alter perceptual effects on a listener.
Moreover, the pitch inflection of voice used for delivery of benefits was wider which could add as a factor of exaggeration and favor the content delivered. Furthermore, the quotients suggest that approximately 50% of the advertisements are fillers with music and description of the hypothetical patient. Based on these measures, the overall perception of an advertisement might have favored the benefits section than the risks. These results suggest the need for standardization in rate of speech for advertisements such that both benefits and risks of medication are perceived equally by the audience.
Future Work
We need to examine the changes in the regulatory environment, i.e. longitudinal study of the effects of FDA regulations on DTC advertisements. The next step would be to conduct a correlation test between the rate of speech and perception of listeners. The findings from this research gave us insight into how DTC advertisements are developed to influence the buying behavior of consumers. We were able to judge whether the risks of drugs are presented faster and less clearly in comparison to the benefits of drugs to suppress negative information that could deter consumption.
Acknowledgement
An earlier version of this research was presented at the Business and Health Administration meeting in Chicago in 2014. This work emerged from the undergraduate research work of the fourth author. The support of the Office of Undergraduate Research of Lamar University is also acknowledged.
References
- Auton, F. (2004). The advertising of pharmaceuticals direct to consumers: a critical review of the literature and debate. International Journal of Advertising, 23(1), 5-52.
- Auton, F. (2006). DIRECT‐TO‐CONSUMER ADVERTISING (DTCA) OF PHARMACEUTICALS: AN UPDATED REVIEW OF THE LITERATURE AND DEBATE SINCE 2003 1. Economic Affairs, 26(3), 24-32.
- Auton, F. (2009). Opinion: the case for advertising pharmaceuticals direct to consumers. Future Medicinal Chemistry, 1(4), 587-592.
- Bell, R.A., Kravitz, R.L., & Wilkes, M.S. (2000). Direct-to-consumer prescription drug advertising, 1989-1998. Journal of Family Practice, 49(4), 329-329.
- Block, A.E. (2007). Costs and Benefits of Direct-to-Consumer Advertising. Pharmacoeconomics, 25(6), 511-521.
- Boersma, P., & Weenink, D. (2014). Praat: doing phonetics by computer [Computer program]. Version 5.3.56, retrieved 20 March 2014 from http://www.praat.org/
- Center of Disease Control and Prevention. (2009). History of the Surgeon General’s Reports on Smoking and Health. Retrieved from http:cdc.gov/tobacco/Data_statistics/sgr/history/index.htm
- Center of Disease Control and Prevention. (2012). Smoking and Tobacco Use Legislation. Retrieved from http://www.cdc.gov/tobacco/data_statistics/by_topic/policy/legislation/
- Chandra, A., & Miller, M. (2005). A Closer Look at the Concept, Historical Overview, and Value of Direct-to-Consumer Advertising of Prescription Drugs. Hospital Topics, 83(4), 32-36.
- Davis, J.J., & Meader, A. (2009). Beyond content analysis: Assessing fair balance in DTC pharmaceutical advertising. Journal of Medical Marketing, 9(1), 57-66.
- Folsom, C., Fesperman, S.F., Tojuola, B., Sultan, S., & Dahm, P. (2010). Direct-to-consumer Advertising for Urological Pharmaceuticals: A Cross-sectional Analysis of Print Media. Urology, 75(5), 1029-1033.
- Frosch, D.L., Grande, D., Tarn, D.M., & Kravitz, R.L. (2010). A decade of controversy: balancing policy with evidence in the regulation of prescription drug advertising. American Journal of Public Health, 100(1), 24-32.
- Frosch, D.L., Krueger, P.M., Hornik, R.C., Cronholm, P.F., & Barg, F.K. (2007). Creating demand for prescription drugs: a content analysis of television direct-to-consumer advertising. The Annals of Family Medicine, 5(1), 6-13.
- Hartgraves, T. (2002). DTC Prescription Drug Advertising: The History and Impact of FDA Regulation.
- Johnstone, T., & Scherer, K.R. (2000). Vocal Communication of Emotion. Handbook of Emotions, Michael Lewis ET Jeannette M. Haviland-Jones. New-York, États-Unis: Guilford, 220-235.
- Mogull, S.A. (2008). CHRONOLOGY OF DIRECT-TO-CONSUMER ADVERTISING REGULATION IN THE UNITED STATES. AMWA Journal: American Medical Writers Association Journal, 23(3), 106-109.
- Mukherjee, A., Limbu, Y., & Wanasika, I. (2013). A review of research on direct-to-consumer advertising of prescription drugs: Directions for future research. International Journal of Pharmaceutical and Healthcare Marketing, 7(3), 226-243.
- Thorsen, A.S. (2009). Analysis of the Risk and Benefit Information Presented in Broadcast Direct-to-consumer Advertising (Doctoral dissertation, University of Georgia).
- U.S. Food and Drug Administration. (2012). Background on Drug Advertising. Retrieved from http://www.fda.gov/Drugs/ResourcesforYou/Consumers/PrescriptionDrugAdvertising/ucm07 1964.htm
- U.S. Food and Drug Administration. (2013). Overview of the Family Smoking Prevention and Tobacco Control Act: Consumer Fact Sheet. [Data File]. Retrieved from http://www.fda.gov/tobaccoproducts/guidancecomplianceregulatoryinformation/ucm246129.
- Ventola, C.L. (2011). Direct-to-Consumer Pharmaceutical Advertising: Therapeutic or Toxic? Pharmacy and Therapeutics, 36(10), 669-684.
- Wilkes, M.S., Bell, R.A., & Kravitz, R.L. (2000). Direct-To-Consumer Prescription Drug Advertising: Trends, Impact, And Implications: Aiming drug ads at consumers means big business for drug companies, but its effect on clinical care is not yet known. Health Affairs, 19(2), 110-128.